AbstractThalidomide has become a novel agent in the treatment of multiple myeloma and many other hematological and solid malignancies. Thalidomide has various adverse effects including neurologic, hematologic, cardiovascular, gastrointestinal, and endocrinologic toxicities. However, with strict regulations for use, there have been few case reports of thalidomide toxicity. A 42-year-old man presented with both breast engorgement and tenderness for 1 year. Six years ago, he had been diagnosed malignant histiocytosis at left palpable axillary mass lesion. He was treated with chemotherapy according to Langerhans cell histiocytosis. But, 6 months later, the response of chemotherapy was refractory and then, he has been received thalidomide salvage therapy (25-100 ㎎/day) for 5 years. Mammography and ultrasonography revealed a bilateral heterogeneous mixed echoic lesion suggesting a fibroglandular tissue under the nipple and subcutaneous layer. We have treated him with bromocriptine. With a follow-up of 15 months, gynecomastia and breast discomforts were improved.
INTRODUCTIONThalidomide (Thalomid,α-[N-phthalimido]-glutarimide) is a glutamic acid derivative that was used in the late 1950s as a therapy for pregnancy-related morning sickness [1]. In 1961, it was quickly taken off the market worldwide after its teratogenicity was recognized [2]. But, over the past 5 years thalidomide has become a novel anticancer agent with antiangiogenic, proapoptotic, and immunomodulatory activities [1,3]. Thalidomide has shown beneficial effect in renal cell carcinoma, Kaposi’s sarcoma, angiogenic myeloid metaplasia, and myelodysplastic syndrome [1,3]. Also, there have been several reports showing its significant effect against histiocytes [4].
Besides teratogenicity, thalidomide has various adverse effects including neurologic, hematologic, cardiovascular, gastrointestinal, dermatologic, and endocrinologic toxicities [1]. We experienced gynecomastia, rare side effect of thalidomide. There has been only one report of thalidomide associated gynecomastia in multiple myeloma similarly. We report here the first case of thalidomide associated gynecomastia in treatment of malignant histiocytosis.
CASE REPORTA 42-year-old man visited our institution due to both breast engorgement and mild tenderness for 1 year (Figure 1A). Six years ago, he had been admitted to our hospital for left palpable axillary mass and diagnosed malignant histiocytosis by punch biopsy. Multiple lymph nodes involvement in the both axilla, both cervical and superior mediastinal lymph nodes were detected by positron emission tomography/ computed tomography (PET/CT). High resolution CT revealed extensive cystic lung disease involving both lungs.
He was then treated with 10 courses of chemotherapy according to LCH (Langerhans cell histiocytosis)-II arm B with vinblastine (6 ㎎/㎡), prednisolone (40 ㎎/㎡), and etoposide (150 ㎎/㎡). But, 6 months later, PET/CT showed multiple residual malignancies with right lower rib metastasis and then, he was started thalidomide salvage therapy (25-100 ㎎/day). Over the 5 years with taking thalidomide, he has been tolerable and experienced no other adverse effects associated with thalidomide. He did not take any other medications that could result in gynecomastia.
Mammography (Figure 1B) and ultrasonography (Figure 1C) revealed a bilateral heterogeneous mixed echoic lesion suggesting a fibroglandular tissue under the nipple and subcutaneous layer. Core needle biopsy was taken and stromal fibrosis was observed (Figure 2). Laboratory findings disclosed normal values of estradiol, testosterone, and β-human chorionic gonadotropin. Serum prolactin was slightly increased to 25.2 ng/mL (1.8-15.9 ng/mL).
Bromocriptine (2.5 ㎎/day) was prescribed for 3 months and thal-idomide was continued unchanged. Three months later serum prolactin was normalized to 2.4 ng/mL and bromocriptine was stopped. With a follow-up of 15 months gynecomastia and both breast discomforts were improved.
DISCUSSIONThalidomide is now commonly used as an anticancer agent after it was approved by the U.S. Food and Drug Administration (FDA) for the treatment of erythema nodosum leprosum in 1998 [1,4]. Currently, several trials are ongoing in other malignant and nonmalignant dis-orders. Given the increasing clinical use of thalidomide, it becomes important to study the adverse effects of this agent. The most serious adverse effects associated with thalidomide is its documented teratogenicity [1]. Other common toxicities are sensorimotor peripheral neuropathy, somnolence, rash, fatigue, and constipation. Less common toxicities include deep venous thrombosis, Stevens-Johnson syndrome, elevated liver enzymes, malaise, and peripheral edema. The incidences of adverse effects are correlates with the dose and duration of therapy. Therapeutic doses of thalidomide are 200 to 400 ㎎/day (4-8 ㎎/㎏/day) for oncological condition, and 100 to 400 ㎎/day (2-8 ㎎/㎏/day) for dermatological and inflammatory disease [3]. Doses of the drug of 200 ㎎/day or less are usually well tolerable [1]. Because of the toxicity, thalidomide is approved for marketing only under a special restricted distribution program approved by the FDA.
Prevention and management of thalidomide toxicity is also important. For women of childbearing potential, effective contraception must be used for at least 4 weeks before beginning thalidomide therapy, during thalidomide therapy, and for 4 weeks following discontinuation of thalidomide therapy. Thalidomide should be discontinued once a complication occurs that is related to the drug. When the complication resolves, thalidomide may be resumed at a 50% dose reduction [1].
To date, the relationship between thalidomide treatment and the development of gynecomastia in malignant histiocytosis has never been reported. In our case, the patient experienced no other adverse effects associated with thalidomide except for gynecomastia. In general, once an adverse effect occurs, thalidomide should be withheld until the toxicity resolves. However, the symptom resolved after medical treatment (bromocriptine 2.5 ㎎/day), thalidomide was continued in this case. We suggest that gynecomastia should be added to the spectrum of thalidomide associated adverse effect. Furthermore, in the treatment of relapsed and refractory disease that has limited therapeutic options, thalidomide may be continued if the symptom that is related to gynecomastia is well controlled with medical treatment.
Figure 1.Clinical feature and radiologic imaging of the patient. (A) Initial presentation shows both breast engorgements (B) Mammography reveals dense mammary tissues with no mammographic evidence of malignancy. (C) Ultrasonography shows a heterogeneous mixed echoic lesion suggesting a fibroglandular tissue. 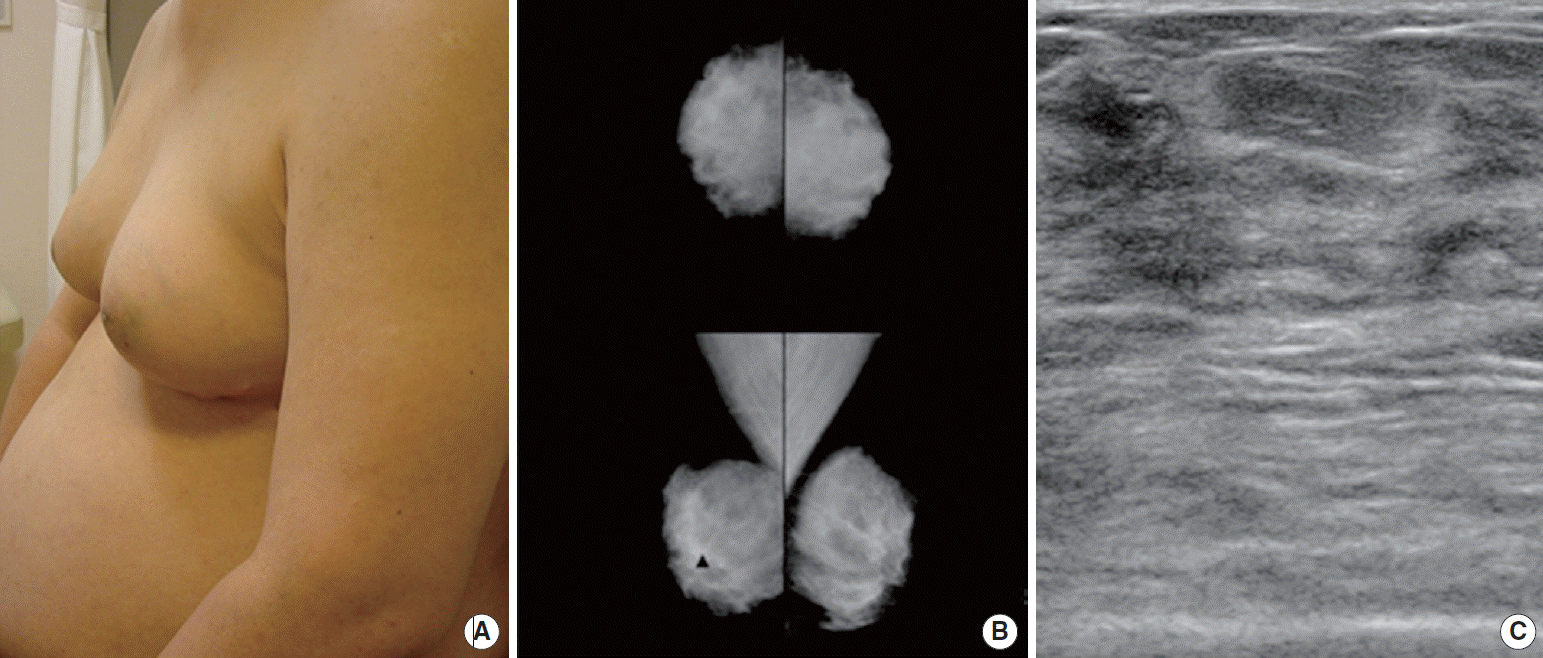
|
|
||||||||||||||||||||||||||||||||