AbstractPurpose:The aim of this study was to compare the incidence of focal thyroid incidentalomas on 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) in breast cancer patients with the incidence in healthy women, and to assess for any significant characteristics of the breast cancers in patients with concurrent focal thyroid incidentalomas.
Methods:We retrospectively reviewed the medical records of 489 breast cancer patients and 286 healthy women who were evaluated with 18F-FDG PET/CT between March 2010 and February 2012. We analyzed the images visually and obtained semiquantitative indices.
Results:The overall incidence of focal thyroid incidentalomas was 9.4% (46/489) in breast cancer patients and 5.9% (17/286) in healthy patients (p<0.01). Among 23 focal incidentalomas in breast cancer patients and five in healthy women with additional cytological and histological diagnoses, papillary thyroid carcinoma was diagnosed in 11 breast cancer patients (23.9%) and in two healthy patients (11.7%). Ductal carcinoma in situ (p=0.03), estrogen receptor (ER) positivity (p<0.01), progesterone receptor (PR) positivity (p<0.01), and Ki-67 positivity (p<0.01) were correlated with breast cancer patients with focal thyroid incidentalomas.
Conclusion:Focal thyroid incidentalomas were more frequently detected with 18F-FDG PET/CT in patients with breast cancer than in healthy women. Focal thyroid nodules incidentally found with 18F-FDG PET/CT in breast cancer patients have a high risk of malignancy. ER positivity, PR positivity, Ki-67 positivity, and ductal carcinoma in situ correlated positively with 18F-FDG PET/CT-detected focal thyroid incidentalomas.
INTRODUCTIONThe overall reported incidence of 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET) or PET/computed tomography (PET/CT)-detected thyroid incidentalomas varies from 0.2% to 8.9%, whereas the presence of thyroid cancer in these incidental lesions can range from 8% to 64% [1,2]. Thyroid incidentalomas may represent benign disease, primary malignancy, or metastases [3]. Both thyroid and breast cancers are more frequent in women than in men, and it has been suggested that estrogen plays a role in the tumorigenesis of both cancers. Several studies have demonstrated that there is an increased risk of breast cancer in patients with thyroid dysfunction and thyroid cancer [4]. As 18F-FDG PET/CT is widely used in the diagnosis, staging, and metastatic workups of patients with neoplastic disease, the incidence of 18F-FDG PET-detected incidentalomas is increasing [5,6]. In Korea, thyroid cancer is the most common female cancer, followed by breast cancer [7]. There have been few studies of 18F-FDG PET/CT-detected thyroid incidentalomas in breast cancer patients [1,8]. Guidelines are needed to manage thyroid incidentalomas detected during treatment for breast cancer.
Our study aimed to evaluate (1) the incidence of focal thyroid incidentalomas identified on 18F-FDG PET for breast cancer compared with the incidence in healthy women; (2) any correlation between the clinicopathological characteristics of breast cancers with concurrent focal thyroid incidentalomas; and (3) the malignant potential of 18F-FDG PET/CT-detected focal thyroid incidentalomas.
METHODSPatient selectionWe reviewed a database of patients and healthy subjects who underwent 18F-FDG PET/CT between March 2010 and February 2012 at our institute; breast cancer patients were enrolled through the breast care center and healthy subjects through our health promotion center. During the study period, 531 female patients with primary breast cancer and 801 healthy subjects were evaluated with 18F-FDG PET/CT. The following exclusion criteria for breast cancer patients or healthy subjects were utilized in this study: (1) male sex, (2) distant metastasis at the time of diagnosis, (3) treatment with neoadjuvant chemotherapy, (4) history of thyroid disease, and (5) previous malignancy. We excluded 42 breast cancer patients and 515 healthy male subjects who met the above exclusion criteria. In total, we analyzed 489 patients with breast cancer and 286 healthy women for this study. The Institutional Review Board of our institute approved this study (129792-2014-021).
18F-FDG PET/CT imaging and imaging analysisAll patients fasted and rested for at least 6 hours before undergoing 18F-FDG PET/CT (Biograph 64; Siemens, Erlangen, Germany) scanning. Serum glucose levels were less than 200 mg/dL before 18F-FDG administration. A total of 4.5 MBq/kg of 18F-FDG was injected into a vein contralateral to the tumor side. At 60 minutes after 18F-FDG injection, a 3-minute static emission study was performed at the transmission position (early phase), another study for the delayed phase was performed 120 minutes after the injection, and whole body imaging was performed from the base of the skull to the mid thighs. Lowdose whole body CT was performed before the emission scan for CT attenuation correction instead of a transmission scan. These data were also used for anatomic localization of the PET emission image. The attenuation-corrected PET images were reconstructed with an ordered subset expectation maximization iterative algorithm. The resulting in-plane image resolution of the axial view had a full width of 5.8 mm at half maximum. Imaging data were obtained with a Biograph Sensation 16 (Biograph 64) scanner, which produces transaxial, coronal, and sagittal reconstructions of CT, PET, and fusion of PET/CT data for interpretation. The Biograph scanner combines a 16-detector-row spiral CT scanner (Somatom Emotion; Siemens) and a high resolution PET scanner with 4.5-mm spatial resolution and three-dimensional image acquisition. A multimodality computer platform was used for image review and manipulation. After scatter and decay correction and reorientation in axial, sagittal, and coronal planes, the 18F-FDG PET/CT images were reviewed by an experienced nuclear physician. All patients were studied in the supine position, oriented slightly obliquely and contralateral to the tumor side. To calculate the maximum standardized uptake value (SUVmax), circular regions of interest (ROI) were manually drawn on the attenuationcorrected emission images throughout the axial planes in which a suspicious lesion could be delineated.
Image analysisOn 18F-FDG PET/CT images, thyroid uptake was considered to be present when there was increased uptake in the thyroid gland compared to the physiological background. For the visual analysis, abnormal FDG uptake was defined as substantially greater activity than in the aortic blood on attenuation-corrected images. Metabolic activity within the thyroid glands was determined. Focal thyroid uptake was defined as uniform distribution of the tracer above the background throughout one lobe. All PET/CT images were read directly from the screen of the computer workstation, and CT findings were recorded for abnormalities, including the heterogeneity of the thyroid parenchyma (homogenous or heterogeneous) and the pattern of calcification (nodular, globular, amorphous, or linear) based on the CT portion of the PET/CT scan. When a space-occupying lesion was present, its exact size and the solid or cystic pattern were confirmed. Semiquantitative evaluation was performed using a Syngo MultiModality Workplace (Siemens AG, Berlin, Germany). A ROI was drawn on the fused PET/CT image to measure the SUV of the tumor. The SUV is defined as follows: (peak kBq/mL in ROI)/(injected activity/g of body weight). To calculate the SUVmax, manually defined circular ROIs were drawn on the attenuation-corrected emission images throughout the axial plane on which a suspicious lesion could be delineated. We defined 18F-FDG PET/CT-detected focal thyroid incidentalomas as the presence of focal FDG uptake in the thyroid including very tiny foci of FDG uptake with a definite nodule on the CT images (Figure 1).
Immunohistochemical staining of breast cancerImmunohistological staining for estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) was performed using arrayed tissue blocks. Briefly, 5 μm-thick, formalin-fixed, paraffin-embedded tissue sections were obtained using a microtome, transferred to adhesive slides, and dried at 62°C for 30 minutes. Sections were incubated, using a benchmark automatic immunostaining device (Ventana Medical Systems, Tucson, USA), with primary antibodies against ER (1:50 dilution; Diona, Seoul, Korea), PR (1:100 dilution; Diona), and HER2 (1:250 dilution; DAKO, Glostrup, Denmark), followed sequentially by incubation with biotinylated antimouse immunoglobulin and peroxidase-labeled streptavidin, using 3.3´-diaminobenzidine chromogenic as the substrate. ER and PR staining were scored using the Allred scoring system on a scale from 0 to 8 for nuclear staining only. The intensity score and percentage score were added together to obtain the Allred score. Allred scores of 0 to 2 were classified as negative and Allred scores of 3 to 8 were classified as positive. HER2 was scored using the current American Society of Clinical Oncology/College of American Pathologist guidelines. Membranous immunoreactivity was scored (0 and 1+ indicates negative; 2+, indeterminate; and 3+, positive for overexpression) and the percentage of tumor cells staining positive was shown as raw score ranging from 0% to 100% [9]. Samples were considered positive for HER2 when strong (3+) membranous staining was observed in at least 10% of tumor cells, whereas those with staining of grades 0 to 2+ were regarded as negative. Tissue samples of HER2 2+ breast cancers underwent silver in situ hybridization analysis. For practical considerations in a hospital setting, as a substitute for gene expression profiling, the use of immunohistochemical surrogate panels of ER, PR, and HER2 has been proposed to potentially differentiate between the subtypes [10].
Cytological confirmation of 18F-FDG PET/CT-detected focal thyroid incidentalomasFine-needle aspiration biopsy (FNAB) is recommended, regardless of the size of the nodule, if it presents sonographic signs suggestive of a malignancy according to the guidelines of the National Cancer Institute [11].
FNAB was performed with a 21-gauge needle on a 10 mL syringe under ultrasound guidance. A cytological diagnosis was made by experienced cytopathologists in our institution. Cytological diagnosis was as follows: (1) nondiagnostic (applies to specimens that are unsatisfactory due to blood, overlaid thick or air-dried smears, or an inadequate number of follicular cells); (2) benign; (3) atypia of undetermined significance (results of proliferating cell nuclear antigen cytology were not easily classified into benign, suspicious, or malignant categories); (4) follicular neoplasm (findings of high cellularity and scant or absent colloid); (5) suspicious for malignancy (only one or two characteristic features of papillary thyroid carcinoma were present, or a malignant diagnosis could not be made with certainty; (6) malignant (whenever the morphologic features were conclusive for malignancy) [12].
Data collectionThe following data were recorded for all breast cancer patients: age, body mass index, number of lymph node metastases, tumor size, histological type, lymph vascular invasion (yes/no), extensive intraductal component (yes/no), p53 expression (yes/no), Ki-67 expression >14% (yes/no), ER status, PR status, HER2 status (positive/negative), thyroid ultrasonographic findings, cytological results of focal thyroid incidentalomas, and SUVmax. We also collected the medical records of healthy women with focal thyroid incidentalomas, such as thyroid ultrasonographic findings, cytological results of the focal thyroid incidentaloma, and SUVmax. We compared the incidence of 18F-FDG PET-detected focal thyroid incidentalomas between patients with breast cancer and healthy women. We compared the clinicopathologic findings between patients with and without 18F-FDG PET-detected focal thyroid incidentalomas.
Statistical analysesThe statistical analyses were performed using SAS version 8.2 (SAS Institute Inc., Cary, USA). Clinical and demographic characteristics were compared using Pearson chi-squared test. SUVmax values were compared using the Mann-Whitney test. All p-values reported are two-tailed, and a p-value of less than 0.05 was considered statistically significant.
RESULTSIncidence of thyroid incidentalomas on 18F-FDG PET/CT: comparisons between primary breast cancer patients and healthy female subjectsIn 489 breast cancer patients, 46 patients (9.4%) had a focal thyroid incidentaloma detected by pretreatment 18F-FDG PET/CT. Seventeen healthy women (5.9%) had an 18F-FDG PET/CT-detected focal thyroid incidentaloma. The incidence of focal thyroid incidentalomas detected on 18F-FDG PET/CT was higher in breast cancer patients than in healthy women (p<0.01).
The clinicopathological characteristics of primary breast cancer patients with an 18F-FDG PET/CT-detected thyroid incidentalomaThe breast cancer patients with focal thyroid incidentalomas (mean age, 52.9 years) was similar to them without focal thyroid incidentaloma (mean age, 51.2 years) (p=0.27). The breast cancer histologic types in the group with thyroid incidentalomas were more often ductal carcinoma in situ compared with the group without thyroid incidentalomas (19.8% vs. 14.5%, p=0.03) (Table 1). The group with thyroid incidentalomas had more ER positivity (56.5% vs. 50.0%), PR positivity (50% vs. 39.3%), and Ki-67 positivity (95.6% vs. 50.2%) than the group without thyroid incidentalomas (p<0.01) (Table 1). HER2 overexpression was higher in the group without focal thyroid incidentalomas than in the group with focal thyroid incidentalomas (23.2% vs. 15.2%, p<0.03). Tumor size, number of metastatic lymph nodes, lymphovascular invasion, extent of the intraductal component, and p53 expression were not different between the two groups (Table 1).
The SUVmax and cytological findings in thyroid incidentalomasAspiration was performed twice for each nodule. FNAB was performed in 23 of 37 focal thyroid incidentalomas (62.1%) in breast cancer and 5 of 12 thyroid incidentalomas (41%) in healthy subjects. The other 21 thyroid incidentalomas were diagnosed as benign nodular hyperplasia by high-resolution thyroid ultrasonography.
In 46 breast cancer patients with focal thyroid incidentalomas, 35 patients underwent thyroid ultrasonography. The results were as follows: six benign, 11 indeterminate, 11 suspected malignancies, and seven diffuse thyroiditis. Among them, 23 patients underwent ultrasound-guided FNAB, resulting in the diagnosis of 12 benign follicular neoplasms and 11 papillary carcinomas (11/46, 23.9%). The mean SUVmax of the focal incidentalomas confirmed as malignant thyroid carcinomas was 7.1±10.7, which was not significantly different from that of the benign focal incidentalomas (7.1± 6.2), (p=0.99). In 17 healthy women with focal thyroid incidentalomas, 12 underwent thyroid ultrasonography, which identified three benign, six indeterminate, and two suspicious malignant nodules. Among them, six patients underwent ultrasound-guided FNAB, resulting in the diagnosis of one benign follicular nodule, three atypia of indeterminate significance, and two suspicious malignant nodules (2/17, 11.7%). All of the malignant thyroid incidentalomas in breast cancer patients and healthy women were diagnosed as papillary thyroid cancer.
DISCUSSIONA focal thyroid lesion with significant uptake is likely to be malignant [13], whereas several benign conditions, including nodular hyperplasia, multiple nodular goiter, benign follicular tumor, and chronic thyroiditis, also show a high SUV. Diffusely increased uptake in the thyroid gland favors several thyroidal disorders, including chronic thyroiditis, Graves’ disease, diffuse goiter, and multinodular goiter [14,15]. A strong relationship between breast malignancy and thyroid autoimmune disorders has been found by Giani et al. [16] and confirmed by Smyth et al. [17]. All of these studies have been carried out in breast cancer patients after mastectomy and before beginning any chemohormonal adjuvant therapy. A high prevalence of antithyroid peroxidase autoantibodies has been found in both treated and untreated breast cancer patients and the positive predictive value of serum thyroid peroxidase antibodies (TPO Ab) in breast cancer patients with aggressive disease has been reported [17].
Only Jiskra et al. [18], despite confirming a higher prevalence of TPO Ab in breast cancer patients, found no impact on relapse-free survival and overall survival; this discrepancy could be due to the relatively small number and heterogeneity of breast cancer patients in their study group. Recently Farahati et al. [19] reported a significantly lower frequency of distant metastases in a large cohort of breast cancer patients with serum TPO Ab positivity. The presence of serum TPO Ab, or clinical hypothyroidism, is associated with longer overall survival in patients with invasive breast carcinoma [20]. Overall, the results of previous studies suggest a positive correlation between thyroid disorders and the risk of developing invasive breast carcinoma [17,21]. On 18F-FDG PET/CT, focal thyroid uptake is incidentally identified in healthy subjects and patients with cancer with an incidence of 0.6% to 3.3%, but in advanced breast cancer patients, Tateishi et al. [22] reported a higher incidence than what was observed in previous studies. Our results in pretreatment breast cancer patients also suggest that a higher incidence of focal thyroid incidentaloma is observed in this patient cohort than in healthy women.
Focal thyroid FDG incidentalomas have been found to have a high risk of being malignant (14%–50%) [1,13,23]. The percentage of malignant thyroid incidentalomas in our study (23.9%) was similar to most previously reported rates [1,12], and lower than other reported data [24]. The current study was a retrospective observation study, and several focal thyroid incidentalomas were no evaluated with FNAB. Because of the high cost of FDG PET and the low incidence of thyroid FDG PET incidentalomas, we cannot use FDG PET prospectively to evaluate the incidence and malignancy rate of thyroid incidentalomas in the general population. A recent study has shown that the CT attenuation pattern of thyroid incidentalomas on dedicated PET/CT could provide additional information for the differentiation of malignant from benign disease [25]. The authors suggested that the 18F-FDG uptake and the CT attenuation pattern, in addition to the SUV analysis, were helpful in characterizing focal thyroid incidentalomas.
There is also much evidence that estrogen has direct effects in thyroid cell lines originating from normal thyroid gland tissue and in thyroid carcinoma by ER-dependent mechanisms, such as the enhancement of proliferation, modulation of the sodium-iodide symporter and thyroglobulin gene expression, and upregulation of matrix metalloproteinase 9 production [26,27]. We developed a hypothesis in which patients with breast cancer were more frequently diagnosed with thyroid cancers than healthy females. Based on the above findings, ER-positive breast cancers, in particular, may be correlated with thyroid carcinoma. We demonstrated that women with primary breast cancer have a higher incidence of focal thyroid incidentalomas than healthy women. We also found that breast cancer with thyroid incidentalomas is most commonly ER-positive breast cancer or ductal carcinoma in situ.
Although several studies [28,29] have investigated the expression of ER subtypes in thyroid cancers without consistent results as of yet, Huang et al. [30] reported that ERα may be a useful immunohistochemical marker for the differential diagnosis of papillary thyroid carcinoma. They also showed an association of the ER subtype expression with Ki-67 positivity and mutant p53 in female papillary thyroid carcinoma patients of reproductive age, suggesting that estrogen-activated ERα may mediate the stimulatory effects on papillary thyroid carcinoma growth and progression; in contrast, ERβ1 has some inhibitory effects. This study was not a prospective study of the relationship between breast cancer and thyroid disease or thyroid malignancy, resulting in a limited ability to estimate these relationships. In addition, further studies are required to evaluate the immunohistochemical markers, such as Ki-67 positivity, ER subtypes, and mutant p53, in both thyroid cancers with focal thyroid uptake incidentalomas and in breast cancer patients with focal thyroid incidentalomas.
In conclusion, this study has demonstrated that focal thyroid incidentalomas are more frequently detected with 18F-FDG PET/CT in patients with breast cancer than in healthy women. Focal thyroid nodules incidentally found with 18F-FDG PET/CT in breast cancer patients have a high risk of malignancy. ER positivity, Ki-67 positivity, and ductal carcinoma in situ correlated positively with 18F-FDG PET/CT-detected focal thyroid incidentalomas. Additional studies need to systematically investigate ER expression in female papillary thyroid cancer patients with concurrent breast cancer and to further analyze the relationship of ER expression with important clinicopathological factors and biological markers (Ki-67, vascular endothelial growth factor) in Korea.
Figure 1.
18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT). (A) Anterior projection, (B) lateral projection, and (C) transaxial planes. A hypermetabolic lesion is seen in the left lobe (blue arrows) of thyroid with standardized uptake value (SUV) of 3.9 and right breast (red arrows) with SUV of 6.0 concurrently. Corresponding CT images of left thyroid incidentaloma (white arrow) was also obtained. 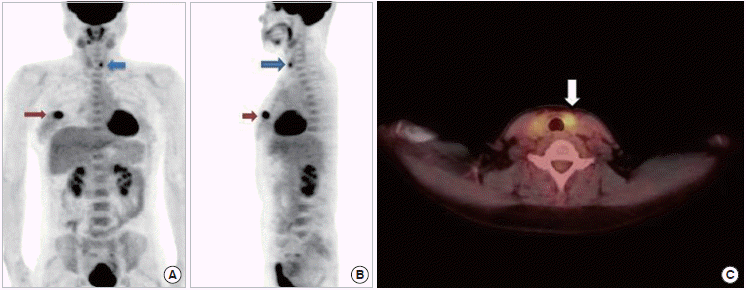
Table 1.Clinicopathological characteristics of breast cancer patients according to 18F-FDG PET/CT thyroid incidentaloma 18F-FDG PET/CT=18F-fluorodeoxyglucose positron emission tomography/computed tomography; BMI=body mass index; LN=lymph node; BCS=breast-conserving surgery; MRM=modified radical mastectomy; IDC=invasive ductal carcinoma; DCIS=ductal carcinoma in situ; LVI=lymphovascular invasion; EIC=extensive intraductal component; ER=estrogen receptor; PR=progesterone receptor; HER2=human epidermal growth factor receptor 2. REFERENCES1. Kang KW, Kim SK, Kang HS, Lee ES, Sim JS, Lee IG, et al. Prevalence and risk of cancer of focal thyroid incidentaloma identified by 18F-fluorodeoxyglucose positron emission tomography for metastasis evaluation and cancer screening in healthy subjects. J Clin Endocrinol Metab 2003;88:4100-4.
2. Ohba K, Nishizawa S, Matsushita A, Inubushi M, Nagayama K, Iwaki H, et al. High incidence of thyroid cancer in focal thyroid incidentaloma detected by 18F-fluorodeoxyglucose [corrected] positron emission tomography in relatively young healthy subjects: results of 3-year follow-up. Endocr J 2010;57:395-401.
3. Prichard RS, Cotter M, Evoy D, Gibbons D, Collins C, McDermott E, et al. Focal thyroid incidentalomas identified with whole-body FDG-PET warrant further investigation. Ir Med J 2011;104:177-9.
4. Kim KJ, Lim H, Kim SY, Hur KY, Park KK, Jang YS, et al. Incidence and characteristics of thyroid nodules in patients with breast cancer. J Korean Breast Cancer Soc 2001;4:115-9.
5. Bomanji JB, Costa DC, Ell PJ. Clinical role of positron emission tomography in oncology. Lancet Oncol 2001;2:157-64.
6. Chen YK, Ding HJ, Chen KT, Chen YL, Liao AC, Shen YY, et al. Prevalence and risk of cancer of focal thyroid incidentaloma identified by 18F-fluorodeoxyglucose positron emission tomography for cancer screening in healthy subjects. Anticancer Res 2005;25:1421-6.
7. Cancer Registry and statistics between 2003-2005. 2008. National Cancer Center of Korea. http://www.ncrr.re.kr. Accessed July 5, 2014.
8. Kim BH, Na MA, Kim IJ, Kim SJ, Kim YK. Risk stratification and prediction of cancer of focal thyroid fluorodeoxyglucose uptake during cancer evaluation. Ann Nucl Med 2010;24:721-8.
9. Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 2007;25:118-45.
10. Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst 2009;101:736-50.
11. Cibas ES, Alexander EK, Benson CB, de Agustín PP, Doherty GM, Faquin WC, et al. Indications for thyroid FNA and pre-FNA requirements: a synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn Cytopathol 2008;36:390-9.
12. Cibas ES, Ali SZ; NCI Thyroid FNA State of the Science Conference. The Bethesda System For Reporting Thyroid Cytopathology. Am J Clin Pathol 2009;132:658-65.
13. Kim TY, Kim WB, Ryu JS, Gong G, Hong SJ, Shong YK. 18F-fluorodeoxyglucose uptake in thyroid from positron emission tomogram (PET) for evaluation in cancer patients: high prevalence of malignancy in thyroid PET incidentaloma. Laryngoscope 2005;115:1074-8.
14. Yasuda S, Shohtsu A, Ide M, Takagi S, Takahashi W, Suzuki Y, et al. Chronic thyroiditis: diffuse uptake of FDG at PET. Radiology 1998;207:775-8.
15. Boerner AR, Voth E, Theissen P, Wienhard K, Wagner R, Schicha H. Glucose metabolism of the thyroid in Graves’ disease measured by F-18-fluoro-deoxyglucose positron emission tomography. Thyroid 1998;8:765-72.
16. Giani C, Fierabracci P, Bonacci R, Gigliotti A, Campani D, De Negri F, et al. Relationship between breast cancer and thyroid disease: relevance of autoimmune thyroid disorders in breast malignancy. J Clin Endocrinol Metab 1996;81:990-4.
17. Smyth PP, Shering SG, Kilbane MT, Murray MJ, McDermott EW, Smith DF, et al. Serum thyroid peroxidase autoantibodies, thyroid volume, and outcome in breast carcinoma. J Clin Endocrinol Metab 1998;83:2711-6.
18. Jiskra J, Barkmanova J, Limanova Z, Lánská V, Smutek D, Potlukova E, et al. Thyroid autoimmunity occurs more frequently in women with breast cancer compared to women with colorectal cancer and controls but it has no impact on relapse-free and overall survival. Oncol Rep 2007;18:1603-11.
19. Farahati J, Roggenbuck D, Gilman E, Schütte M, Jagminaite E, Seyed Zakavi, et al. Anti-thyroid peroxidase antibodies are associated with the absence of distant metastases in patients with newly diagnosed breast cancer. Clin Chem Lab Med 2012;50:709-14.
20. Giustarini E, Pinchera A, Fierabracci P, Roncella M, Fustaino L, Mammoli C, et al. Thyroid autoimmunity in patients with malignant and benign breast diseases before surgery. Eur J Endocrinol 2006;154:645-9.
21. Franceschi S, la Vecchia C, Negri E, Parazzini F, Boyle P. Breast cancer risk and history of selected medical conditions linked with female hormones. Eur J Cancer 1990;26:781-5.
22. Tateishi U, Gamez C, Dawood S, Yeung HW, Cristofanilli M, Inoue T, et al. Chronic thyroiditis in patients with advanced breast carcinoma: metabolic and morphologic changes on PET-CT. Eur J Nucl Med Mol Imaging 2009;36:894-902.
23. Cristofanilli M, Yamamura Y, Kau SW, Bevers T, Strom S, Patangan M, et al. Thyroid hormone and breast carcinoma: primary hypothyroidism is associated with a reduced incidence of primary breast carcinoma. Cancer 2005;103:1122-8.
24. Cohen MS, Arslan N, Dehdashti F, Doherty GM, Lairmore TC, Brunt LM, et al. Risk of malignancy in thyroid incidentalomas identified by fluorodeoxyglucose-positron emission tomography. Surgery 2001;130:941-6.
25. Choi JY, Lee KS, Kim HJ, Shim YM, Kwon OJ, Park K, et al. Focal thyroid lesions incidentally identified by integrated 18F-FDG PET/CT: clinical significance and improved characterization. J Nucl Med 2006;47:609-15.
26. Santin AP, Furlanetto TW. Role of estrogen in thyroid function and growth regulation. J Thyroid Res 2011;2011:875125.
27. Kamat A, Rajoria S, George A, Suriano R, Shanmugam A, Megwalu U, et al. Estrogen-mediated angiogenesis in thyroid tumor microenvironment is mediated through VEGF signaling pathways. Arch Otolaryngol Head Neck Surg 2011;137:1146-53.
28. Di Vito M, De Santis E, Perrone GA, Mari E, Giordano MC, De Antoni E, et al. Overexpression of estrogen receptor-α in human papillary thyroid carcinomas studied by laser- capture microdissection and molecular biology. Cancer Sci 2011;102:1921-7.
|
|
||||||||||||||||||||||||||||||||