INTRODUCTION
Breast cancer is the most common malignancy in women, and ductal carcinoma In Situ (DCIS) is considered as a precursor of breast cancer [1]. Follicular lymphoma (FL) is the second most common form of non-Hodgkin lymphoma (NHL), which is a lymphoma of follicle center B cells. The frequency of FL in Asian countries is approximately 10% [2]. Previously, cases of breast cancer had been reported after the treatment of hematologic malignancies, such as Hodgkin’s disease; however, the synchronous occurrence of breast cancer and lymphoma has been rarely reported [3]. Synchronous presentation of DCIS of the breast and FL is even more rare [4]. Herein, we presented a case where both pathologies coexisted without any history of previous therapy. In addition, a literature review was conducted, and the etiology and management of synchronous breast cancer and FL were discussed.
CASE REPORT
A 49-year-old woman presented with palpable lumps in both axilla a week prior to visiting the hospital. She had significant swelling on both axilla. No family history of breast cancer or lymphoma was reported. Nonspecific induration was palpated on the upper inner quadrant of the right breast. Enlarged lymph nodes were observed in both axilla, which were correlated to swelling. Results of the physical examinations of the left breast, neck, abdomen, and both inguinal area were not significant.
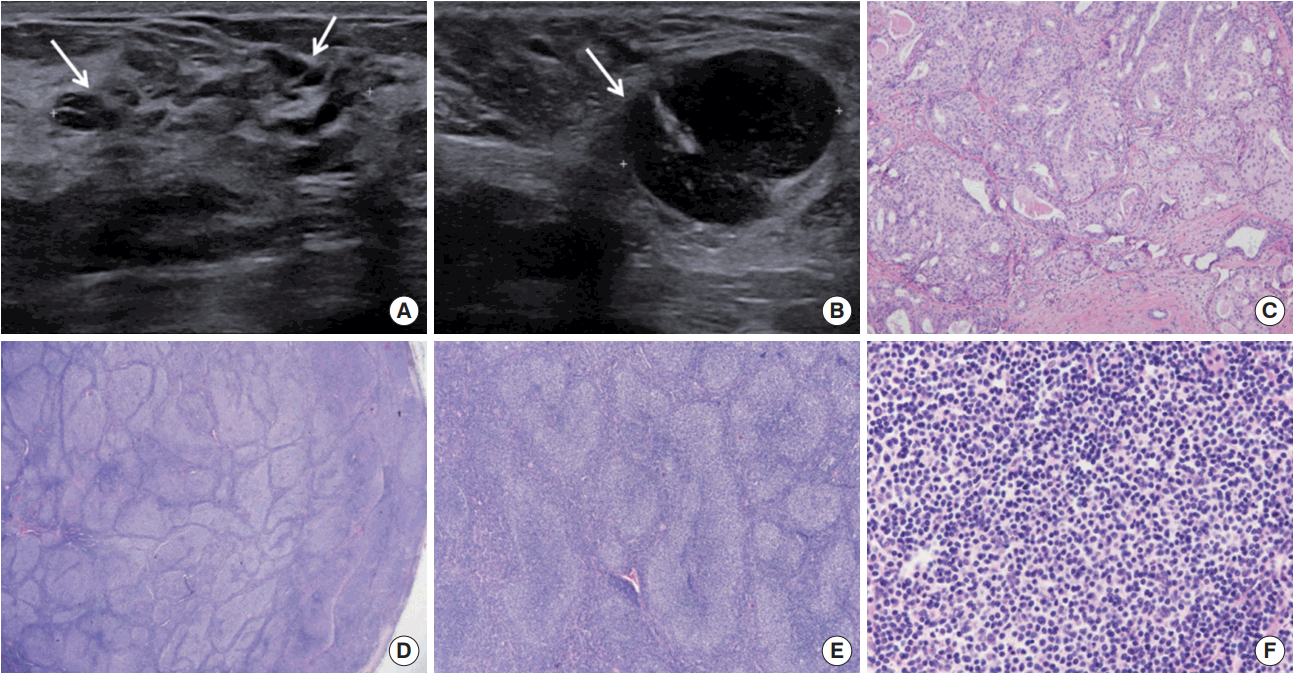
Ultrasonography (US) (Philips Medical Systems, Bothell, USA) examination revealed diffused multifocal segmental duct dilatation with hypoechogenicity in the upper inner quadrant of the right breast (Figure 1A) and multiple enlarged lymph nodes with severe cortical thickening in both axilla (Figure 1B). The breast lesion was classified as C4b based on Breast Imaging Reporting and Data System.
US-guided core needle biopsy (CNB) of the right breast lesion revealed intraductal papilloma. Fine-needle aspiration biopsy of both axillary lymph nodes showed scattered lymphoid cells, and CNB was subsequently conducted for tissue diagnosis. In addition, CNB of the enlarged lymph nodes in the right axilla revealed atypical lymphoid hyperplasia. She underwent excisional biopsies of both the right breast lesion and axillary lymph node.
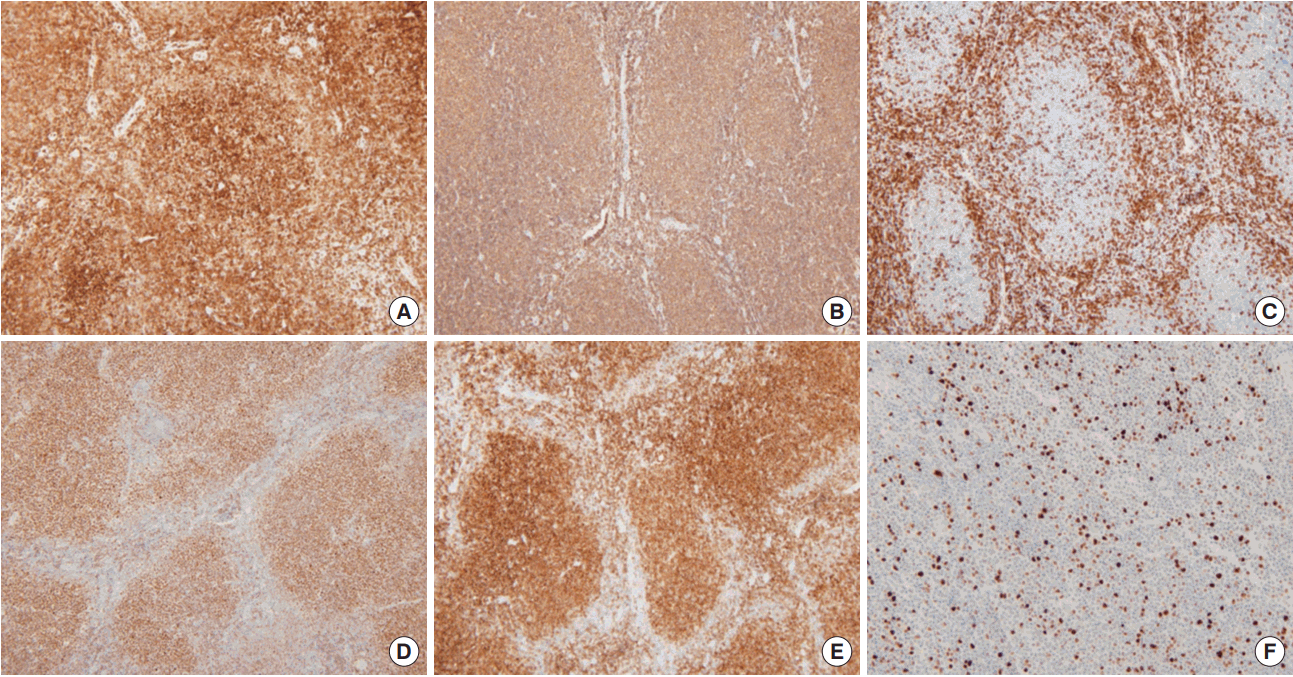
A histopathological examination of the right breast lesion showed intermediate nuclear grade DCIS with a solid and cribriform architectural pattern, which was 0.5× 0.4× 0.4 cm in size (Figure 1C). The right axillary lymph node was a low-grade FL (Figure 1D-F), which was positive for cluster of differentiation 20 (CD20), CD10, CD3, B-cell lymphoma 2 (BCL-2) protein, and BCL-6 protein, but negative for cyclin- D1. Ki-67 was 5% (Figure 2). The lymph node was classified as stage III based on the American Joint Committee on Cancer (AJCC).
Positron emission tomography/computed tomography (PET/CT) (Discovery ST or STE PET/CT; General Electric Medical Systems, Milwaukee, USA) scans showed mild 18F-fluorodeoxyglucose (18F-FDG) uptake in the right breast as well as in both axillary lymph nodes, right supraclavicular area, left upper back area, and intra-abdominal lymph nodes along the right common iliac vessel (Figure 3).
She underwent lumpectomy. Histopathologic evaluation of the breast specimen was also performed, and all margins were negative for cancer. Immunohistochemical staining of the DCIS was positive for estrogen receptor (Allred score, 7), progesterone receptor (Allred score, 7), and human epidermal growth factor receptor 2 protein expression. The AJCC stage was pTispN0M0, stage 0.
Her local therapy was completed along with radiation therapy (RT) of the right breast, and adjuvant hormonal therapy (tamoxifen) was continued. She was scheduled for six cycles of chemotherapy consisting of cyclophosphamide, vincristine, adriamycin, and prednisone.
DISCUSSION
An increased risk of breast cancer has been reported in patients with lymphoma who have been treated with RT [3]. Few articles about secondary lymphoma after adjuvant RT or chemotherapy for breast cancer are also available [5]. However, these two diseases rarely coexist. Two or more primary carcinomas can coexist upon diagnosis or develop consequently, occasionally years after the resection of the first primary tumor. According to the Surveillance, Epidemiology, and End Results cancer registry, women with a history of breast cancer have a 12% reduced risk of developing NHL [6].
Several studies on the coexistence of DCIS and FL and its etiology are available. Suresh et al. [7] presented a case of breast cancer that coexisted with B-cell FL and discussed that the antigenic stimulation of the adjacent carcinoma, such as that in breast cancer, may have induced lymphoma genesis. This mechanism is similar to the role of Helicobacter pylori on inflammatory response, which causes stomach mucosa-associated lymphoid tissue (MALT) lymphoma. Stomach MALT lymphoma may be caused by continuous antigenic stimulation from H. pylori infection [8].
The association between these two diseases could also be explained by a common genetic background or the effects of epidemiologic factors. Ataxia telangiectasia mutated genes, which are observed in lymphoid and breast neoplasms, could be another factor [4]. Wiernik et al. [9] proposed that the mouse mammary tumor virus (MMTV)-like Env gene can be another cause. These gene sequences were detected in more than one-third of the breast cancer study population, and these sequences were also found in two patients with simultaneous presentation of breast cancer and lymphoma. Moreover, both breast cancer and lymphoma may be caused by an MMTV-like retrovirus.
Finally, even though it was not clear which malignancy—lymphoma or breast cancer—developed first, primary cancer-related immunological impairment predisposes secondary malignancies [10]. Lymphoma may block the flow in the lymphatic vessels and subsequently alter the pathway of metastasis in patients with breast cancer. Furthermore, neoplastic lymphoid cells could decrease interleukin-induced adhesion or the tissue necrosis factor of breast cancer cells to the endothelial layer of the axillary lymph nodes, promoting metastasis to certain lymph nodes [11].
In summary, the synchronous presentation of DCIS and FL is rare and difficult to diagnose. As in our case, the diagnosis of lymphadenopathy as a lymphoproliferative disease rather than breast metastasis was challenging. Multiple enlarged axillary lymph nodes on both sides that are found during physical examination or imaging studies should be meticulously diagnosed, particularly in patients with no signs of abnormal breast lesions. Such lymphadenopathy could prevent other malignancies or inflammatory disorders rather than metastatic diseases. Histopathologic confirmation of the synchronous presentation of both breast cancer and axillary lesions is indicated. Thus, clinicians must acknowledge that two different malignancies can coexist, and the recognition of the coexistence can help in the development of treatment plans for both diseases in the future.