INTRODUCTION
Idiopathic granulomatous mastitis (IGM) is a rare type of mastitis that was first described by Kessler and Wolloch [1], and is characterized by chronic, noncaseating granulomatous inflammation of breast lobules. Kessler and Wolloch [1] and Fletcher et al. [2] reported that the most common clinical presentation is a palpable lobular granulomatous inflammation in the breast with or without pain. The size of the mass varies from 0.5 to 9.0 cm; in some cases, nipple retraction or skin fistulas are observed. Moreover, Jorgensen and Nielsen [3] reported axillary lymph node enlargement in 15% of patients of IGM. All affected patients are typically of childbearing age and have a history of oral contraceptive use [1,2,4]. Antibiotics and corticosteroids or surgery have been reported as treatment options.
Despite its benign nature, IGM is often misdiagnosed as breast cancer because of certain clinical and radiological similarities; therefore, a pathologic diagnosis conducted via biopsy is essential. Moreover, IGM is pathologically characterized by chronic and lobular mastitis with idiopathic, noncaseating granuloma, but often, the histologic findings overlap with those of granulomatous mastitis caused by other etiologies such as mycobacterial infections. Therefore, serological and histological examinations are necessary for achieving a differential diagnosis [3]. IGM is a rare disease and its pathogenesis remains controversial; hence, it can be misdiagnosed at a local clinic, and proper treatment may thus be delayed. In this study, we retrospectively reviewed data for 43 patients with IGM who were diagnosed, treated, and monitored between January 2005 and December 2016.
METHODS
Herein, a review of medical records from Breast Cancer Center of Gachon University Gil Medical Center for the period of January 2005 to December 2016 resulted in the identification of 43 patients with a pathologically confirmed diagnosis of IGM who were treated and followed up.
Patient information such as medical history, social history and clinical symptoms was collected through initial interviews; subsequently, physicians performed a physical examination. Mammography was performed using a Selenia (Hologic Inc., Bedford, USA) full field digital mammography unit for women over the age of 30 years, except for those unable to undergo the procedure because of pain or pus drainage. Ultrasonography was performed on all patients by radiologists and the size of the lesion was defined as the major axis length as determined via ultrasonography. At the time of examination, breast lesions were evaluated and enlargement of axillary lymph nodes was confirmed.
To establish a pathophysiologic diagnosis, three to five specimens were collected using via fine needle aspiration (FNA), core needle biopsy (CNB), or both. FNA was performed using a 20 mL syringe with a 20-gauge needle, and the CNB was performed using an ultrasonogram and an automatic biopsy gun equipped with a 14-gauge needle under lidocaine local anesthesia.
The tissues obtained via FNA or CNB were stained with hematoxylin and eosin, Gomori methenamine silver, and the Gram stain, as well as for acid-fast bacilli, using paraffin-embedded sections. Samples were cultured to exclude infectious causes due to bacteria as well as tubercular bacilli.
RESULTS
Herein, 41 of the 43 patients (95.34%) were of childbearing age, ranging from 21 to 50 years, with a median age of 36 years. Of the 43 patients, seven (16.27%) were breastfeeding prior to the diagnosis of IGM and five (11.62%) had a history of oral contraceptive use. Six patients (13.95%) were receiving medication for schizophrenia or bipolar disorder, and two (4.65%) were receiving medication for diabetes. Thirty-two patients (74.41%) were diagnosed with simple mastitis and were treated with antibiotics or incisional drainage.
Thirty-two patients (74.41%) visited Gachon University Gil Medical Center via the local clinic. Physical examinations revealed 21 (48.83%) cases of IGM of the right breast, and 21 (48.83%) of the left breast, while one patient had multiple lesions affecting both breasts. Twelve patients (27.90%) had a body mass index in the normal range (< 23 kg/m2) at the time of diagnosis, nine (20.93%) were overweight (23–24.9 kg/m2), and 22 (51.16%) were obese (≥ 25 kg/m2).
In order to obtain a definite diagnosis, all patients underwent pathological examination using FNA or CNB specimens. Bacteria were detected in two patients; coagulase-negative Streptococcus in one and Streptococcus group C in the other. All specimens were stained for acid fast bacilli to check for the presence of tuberculosis; however, all tests yielded negative results.
All patients received steroid therapy, surgical resection, or both. Patients were monitored on an outpatient basis at intervals of 1 to 2 weeks in the clinic until the lesion disappeared after treatment completion, follow-up visits were scheduled twice at 6-month intervals. Corticosteroids were administered to 36 patients (83.72%); the other seven patients (16.27%) underwent surgery immediately after diagnosis. Corticosteroid treatment was initiated at a dose of 0.4 mg/kg/day. No patient experienced any side effects such as glucose intolerance or iatrogenic Cushing’s syndrome. Although the steroids were administered for more than 6 weeks, nine patients did not respond to treatment. Eighteen of the 43 study patients (41.86%) underwent only nonsurgical treatment. In 24 patients (55.81%), partial mastectomy was performed for lesion removal. Only one patient underwent total mastectomy owing to multiple lesions and steroid resistance.
Twelve patients (27.90%) developed recurrence, but there was no evidence of recurrence in the other 31 patients. Eight (66.6%) patients developed ipsilateral recurrence and four contralateral recurrence (Table 1).
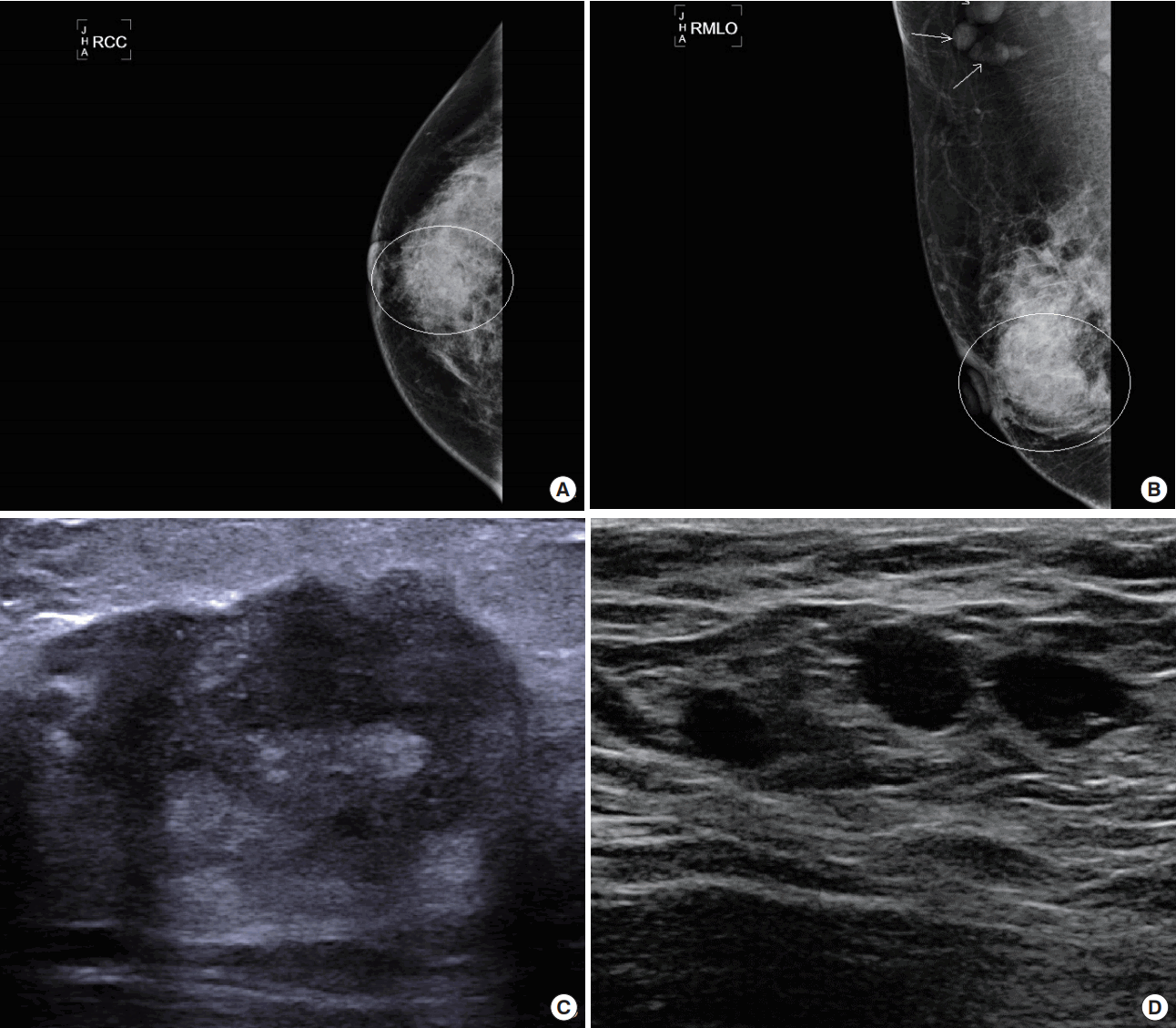
Mammography was performed in 28 patients over the age of 30 years. Of these, 15 (53.57%) showed focal asymmetric hyperdense lesions (Figure 1A) and seven (25.00%) showed ill-defined masses (Figure 2A). In five patients (17.85%), mammography revealed no specific findings other than a dense breast (Figure 1B). Two patients (7.14%) had focal skin thickening (Figure 2B) and six patients (21.42%) exhibited axillary lymph node enlargement (Table 2).
The most common ultrasonographic finding (22 patients, 51.16%) was an ill-defined, heterogeneous, hypoechoic lesion (Figure 1C, D). Sixteen of the study patients (37.20%) had an irregular hypoechoic mass (Figure 2C). In three patients, a tubular, hypoechoic lesion was observed; in two patients, only parenchymal distortion was observed. In nine patients (20.93%), the perilesional fat tissue showed increased echogenicity and in two patients (4.65%), accompanying duct dilatation was observed. In three patients (6.97%), skin thickening was evident (Figure 2D). Upon ultrasonography, 24 patients (55.81%) did not show axillary lymph node enlargement, 16 patients (37.20%) had enlarged benign lymph nodes and three patients (6.97%) had an enlarged lymph node suspicious of malignancy.
According to the Breast Imaging-Reporting and Data System (BIRADS), 13 patients (30.23%) demonstrated category 3 lesions and the remaining 30 (69.77%) demonstrated category > 4A lesions; that is, these cases could be considered as suspected malignancy (Table 3).
DISCUSSION
Although several cases of IGM of unknown origin and with nonspecific clinical features have been reported, its diagnosis and treatment have remained controversial. The underlying pathogenesis of IGM is unknown, though autoimmune disease, undetected microorganisms, postnatal reactions, and the effect of oral contraceptives have been suggested as possible causes [1,5]. Fletcher et al. [2] suggested that the epithelium of lactiferous ducts damaged by infection, trauma or a chemical-induced inflammation would allow ductal secretion leakage into the lobular connective tissue, and leads to lobular structural damage simulating granulomatous reactions.
Lin et al. [6] reported that some patients with IGM are parous and also detected hyperprolactinemia in some patients. In our study, almost all patients were of childbearing age and some patients had a history of lactation or oral contraceptive use, which can suppress lactation. Moreover, Lin et al. also reported that IGM is associated with psychiatric drug induced hyperprolactinemia. In our study, six patients (13.95%) were treated with psychiatric medication, but no evidence to support this findings, via serologic tests, was observed at the time of diagnosis. Nevertheless, one of our patients had hyperprolactinemia and her prolactin level was 108.84 ng/mL. Another patient was diagnosed with hyperprolactinemia and prolactinoma of the pituitary gland at 1 year after treatment completion, and at that time her prolactin level was 88.90 ng/mL.
The most common symptom of IGM is a palpable mass, predominantly unilateral; bilateral lesions are rarely observed [3]. Occasionally the mass is accompanied by pain, skin thickening, fistulas, or axillary lymphadenopathy [7].
Biopsy is essential for the diagnosis of IGM which allows for its distinction from breast cancer or granulomas of other origins on the basis of typical pathologic findings [1-4]. In our study, specimens were collected using FNA, CNB or both. The procedure was determined according to the discretion of the practitioner and the time of diagnosis. Therefore, a difference in diagnosis rates according to each method can be anticipated. Thirty patients (69.77%) were found to have BI-RADS category > 4A lesions, as assessed via mammography and ultrasonography. This is in accordance with the finding of previous studies showing that IGM could not be diagnosed on the basis of radiologic findings alone.
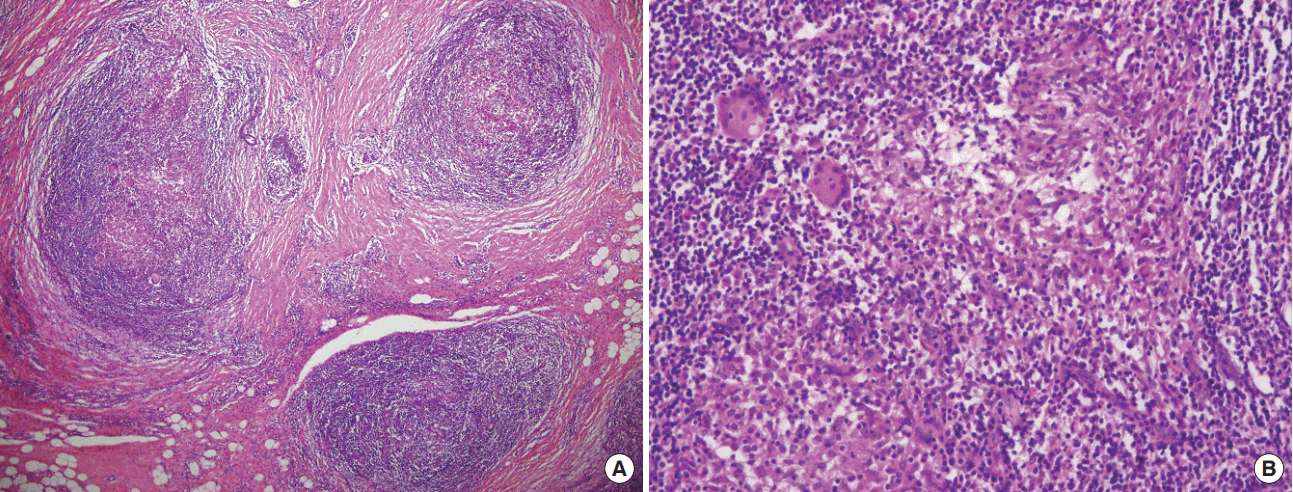
The pathological features of IGM include chronic granulomatous inflammation without necrosis (Figure 3A). Granulomas typically contain lymphocytes, plasma cells, epithelioid histiocytes, multinucleated giant cells, and rarely neutrophils (Figure 3B); granulomatous lesions can be observed in breast lobules or terminal ducts in any breast quadrant except subareolar region [3]. Infectious causes of granuloma of the breast include corynebacterial infection, tuberculosis, cat-scratch disease, and fungal infection. Noninfectious causes include sarcoidosis, Wegener’s granulomatosis, giant cell arteritis, polyarteritis nodosa, ductal ectasia, foreign body reaction, and granulomatous reactions due to breast cancer [8-10]. Infectious causes should be diagnosed differentially, taking into account the corticosteroid usage. Tuberculosis arising from the breast is a rare disease, but the incidence of breast tuberculosis as a complication of acquired immunodeficiency syndrome is increasing worldwide [11]. Moreover, when women of childbearing age complain of a palpable breast mass in a geographic area where tuberculosis is endemic (such as Korea), the possibility of breast tuberculosis should not be overlooked. Breast tuberculosis and other infectious causes can be excluded via serologic and pathologic testing, for example, by using bacterial and fungal cultures and specific stain-based methods [12-14].
There are still no diagnostic criteria or treatment guidelines for IGM. Before the 1980s, complete resection of the lesion was the only treatment applied [9]. Since DeHertogh et al. [15] first described the usefulness of corticosteroid treatment, it has been considered a therapeutic option, and although effective, high doses cause side effects such as glucose intolerance or Cushing’s syndrome. In recent studies, low-dose steroids have been recommended as first-line therapy [6,11], and although extensive surgical resection is still recommended by some physicians, corticosteroid administration may be helpful in avoiding the need for surgery. Initially, as a result of a study performed by DeHertogh et al. [15] in the 1980s, high-dose steroid treatment was routinely administered, the well-known side effects of steroids were noted and lower doses were recommended [2,6-8,13]. At our center, we administered steroids at an initial dose of 0.4 mg/kg/day. All patients underwent steroid treatment without any side effects, and 18 of 43 steroid-treated patients did not require surgery. The steroid dose was tapered for 1 to 28 weeks depending on symptom improvement or changes in clinical features. If recurrence occurs after mastectomy due to a poor response to steroids, reoperation or high-dose steroid treatment may be effective in cases of delayed relapse or complete recovery [11]. In addition, in one study, methotrexate was found to be a favorable option for patients with recurrence or steroid-resistance [16].
IGM is rare and is sometimes misdiagnosed as simple mastitis or breast cancer. However, when diagnosed timely through radiologic and histologic examination, and on the basis of low-dose corticosteroid usage, clinical outcomes can be improved without the need for surgical treatment. Delayed diagnosis and treatment could result in unnecessarily prolonged pain, abscess or fistula formation, and cosmetic impairments. In view of the fact that almost all patients with IGM are young and socially active women, this is not only a personal problem, but also a socioeconomic one. In conclusion, we recommend that studies be undertaken to determine the etiology of IGM and establish rapid and appropriate diagnostic and therapeutic protocols.