INTRODUCTION
Breast hamartoma is an uncommon benign disease of the breast, accounting for 4.8% of all benign breast masses [
1]. Hamartomas comprise varying proportions of fat, fibrous, and adenomatous elements, which result in different radiologic and pathologic findings. Thus, hamartomas are easily confused with other well-circumscribed, benign breast diseases such as phyllodes tumors and fibroadenomas. Moreover, breast hamartoma is usually found unilaterally, ranging in size from 0.9 to 6.9 cm [
1].
Herein, we report an unusual case of huge bilateral breast hamartoma with hamartoma in axillary ectopic breast tissue.
CASE REPORT
A 34-year-old woman visited Breast Center of Inje University Busan Paik Hospital for the evaluation of hard bilateral breast masses; the right and left masses were 25 cm and 18 cm in size, respectively (
Figure 1A). She had no medical history, and her family did not have any breast-related medical history. The patient was not married and had no children. The patient’s previous breast size was A cup, but her breasts had gradually enlarged to the aforementioned size in only 6 months. She also had a mass on her left axilla.
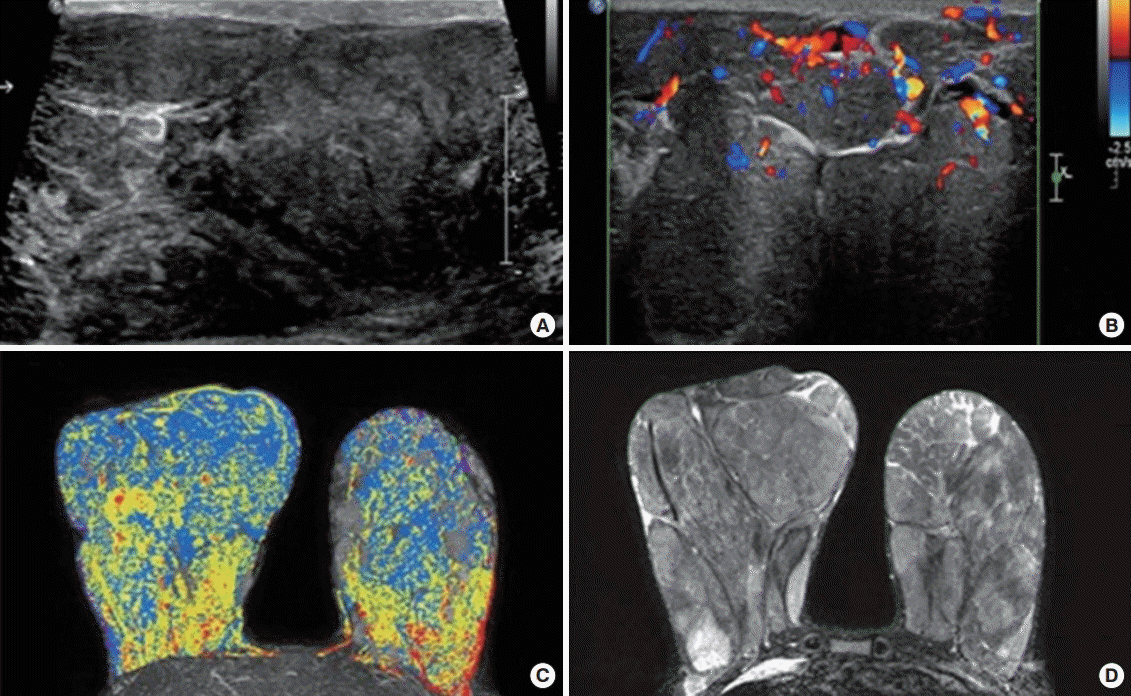
The masses were a conglomeration of multiple well-defined heterogeneously hypoechoic masses, occupying almost the entirety of both breasts on ultrasonography. Some of the masses had internal slit-like anechoic spaces that are frequently found in phyllodes tumors or pseudoangiomatous stromal hyperplasia (PASH). Markedly increased vascular signals in the masses were seen on color Doppler images. A well-circumscribed hypoechoic mass showing ultrasonographic features similar to those of the breast masses was also seen in the left axilla. Normal breast parenchymal tissue was not observed. The ultrasonographic pattern was suggestive of multiple fibroadenomas, phyllodes tumor or PASH (
Figure 2A,
B).
Huge well-circumscribed, soft tissue masses of heterogeneous signal intensity were seen in both breasts on axial T1/T2-weighted magnetic resonance imaging (MRI) (
Figure 2D). Sagittal dynamic contrast-enhanced MRI showed strong heterogeneous multilobular enhancing masses that resembled orange slices. Time-intensity analysis indicated early rapid initial rise and washout kinetics. Diffusion-weighted MRI showed multifocal, multicentric high signal intensity. These MRI findings suggested the possibility of malignancy (
Figure 2C,
D). The kinetic features of the tumor were attributable to the increased vascularity of the masses, as seen on color Doppler ultrasonography.
Needle biopsy of the right breast mass (12 o’clock site) was suggestive of either a fibroepithelial tumor or PASH. The possibility of phyllodes tumor accompanying malignancy could not be ruled out, even though the results of the needle biopsy suggested benign diseases.
The patient underwent bilateral mastectomy followed by immediate implant reconstruction (
Figure 1B). In order to ensure safety margin of the tumor, subcutaneous mastectomy including overlying skin excision was done. Excised right and left breast masses were 25×19 cm and 21×18 cm in diameter, respectively. Cut sections of both breast masses showed similar features with multinodular appearance and focal fibromyxoid areas. The left axillary mass measured 5.5×4.4 cm, with yellowish white nodules on the cut section (
Figure 3). The masses on the right and left breasts weighed 1,980 g and 1,233 g, respectively, while the mass on the left axilla weighed 36 g.
Microscopically, both breast masses exhibited similar histologic features with relatively well-demarcated borders. Benign-looking breast parenchymal tissue showed multinodular growth, with focal PASH (left-sided mass) and lymphangiectasia (right-sided mass) in the stromal tissue. Pathologically, the patient was diagnosed with mammary hamartoma. Immunohistochemical staining of the left-sided mass revealed that the cells were positive for CD34, focally positive for epidermal growth factor receptor (EGFR) and negative for smooth muscle actin. The excised left axillary mass was diagnosed as hamartoma arising from the accessory breast tissue (
Figure 4).
The results of the hormone essays were as follows: estradiol, 290 pg/mL; follicle-stimulating hormone, 2.02 mlU/mL; prolactin, 9.16 ng/mL; and progesterone, 13.02 ng/mL. All these results were within normal ranges. Twelve months after the surgery, the patient has not experienced any complications or tumor recurrence.
DISCUSSION
We performed surgery for an unusual case of macromastia confirmed as bilateral breast hamartoma, with hamartoma in axillary ectopic breast tissue.
The definition of macromastia or gigantomastia varies, but the most common definition is breast enlargement that requires breast reduction surgery to remove >1,800 g of tissue on each side [
2]. Differential diagnosis for breast hypertrophy includes virginal hypertrophy, gravid hypertrophy, fibroepithelial tumors, traumatic hypertrophy, and pseudohypertrophy due to obesity, in addition to endocrine abnormalities [
3]. Because hamartoma is an excess of normal tissue, our patient’s cause of breast hypertrophy is thought to be due to hamartoma itself. The reason the patient’s breasts had markedly enlarged from A cup-sized breasts to gigantomastia within only 6 months is still unclear. However, because her estrogen and progesterone levels were within normal ranges, and the immunohistochemistry stain for EGFR was focally positive, it could be speculated that her breast parenchyma had become hypersensitive to estrogen, progesterone or certain growth factors.
Breast hamartoma is an uncommon benign breast disease, and its pathogenesis is yet to be elucidated. Most breast hamartomas are found unilaterally; very few cases of bilateral breast hamartomas have been reported, and, to date, no case of bilateral breast hamartoma with hamartoma in ectopic breast tissue has been reported [
4]. Sizes of hamartomas vary from as small as a few centimeters to 25 cm. The conglomeration of radiologic and pathologic findings is important in the diagnosis of hamartomas. Depending on the varying proportions of fat and fibroglandular tissue, radiologic studies show inconsistent traits, making diagnosis difficult. It is widely known that the diagnostic value of needle biopsy in breast hamartoma is very limited, often misdiagnosing as fibroadenoma or other benign diseases [
5]. Therefore, complete excision of hamartoma is imperative in the diagnosis.
Radiologically, hamartomas have a mammographic appearance typical of lucent lesions containing fat, varying proportions of radiodense fibrous and adenomatous elements, sharp margins, and in some cases, a thin capsule [
6]. The role of ultrasonography in the diagnosis of hamartomas is limited because hamartomas may have a wide range of ultrasonographic appearances. In a study by Chao et al. [
7], breast hamartomas are well-circumscribed, solid, oval tumors without intratumoral microcalcification. The internal echo texture of most hamartomas is either hyperechoic or of mixed echogenicity. They are often described as a “slice of salami” [
8]. Characteristic MRI findings of hamartomas include heterogeneous intensities on conventional T1- and T2-weighted MRI, which correlate with their fatty, fibroglandular contents and thin capsule [
5].
Pathologically, a definite diagnostic criterion for hamartoma is still lacking; however, a hamartoma consists of a mixture of benign epithelial elements, fibrous tissue, and fat. Normal compressed tissue along the periphery of the mass is another significant pathologic finding [
7]. Our case was diagnosed as hamartoma with PASH. PASH was first described by Vuitch et al. [
9] in 1986, indicating a benign breast disease with focal proliferation of fibroblast and myofibroblast cells. The breast stromal tissue contains vessel-like structures with increased collagenous tissue [
10]. However, there are no red blood cells in these vessel-like structures, and spindle cells, without atypia, are found; along this space, these spindle cells are positive for vimentin, CD34, actin, and desmin but negative for cytokeratin or factor VIII-related antigens. Additionally, fibroblast-like features are observed on electron microscopy. The presence of these vessel-like cavities with spindle cells means that PASH is often mistaken for low-grade angiosarcoma. Although spindle cells are factor VIII-related antigen-negative, it is difficult to diagnose PASH if the pathologist is unaware of its clinical and pathologic features [
11].
In cases with huge benign breast masses, surgeons must consider that, although needle biopsy may suggest fibroadenomas or other tumors, final diagnosis may be otherwise. In cases such as the one reported here, when breast mass is huge and the pathology of needle biopsy is ambiguous, surgery is mandatory for both the confirmatory diagnosis and for the proper management of the tumor, as well as to fulfill the patient’s cosmetic needs.