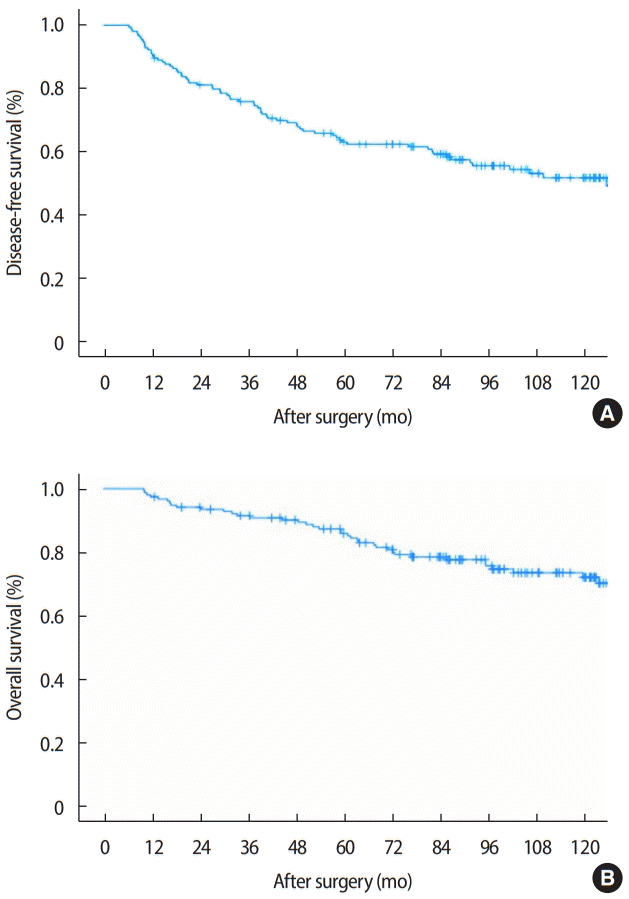
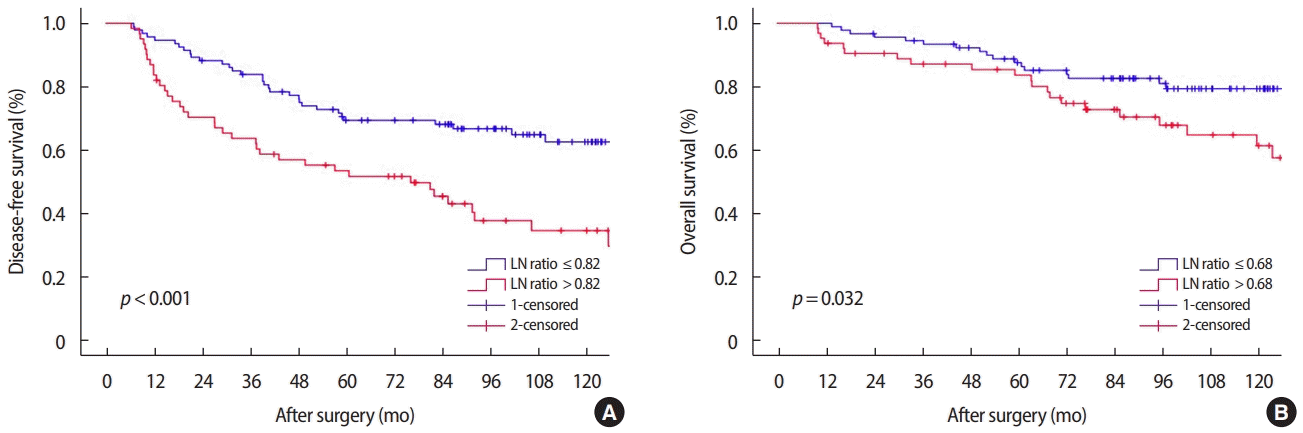
3. Koca E, Kuzan TY, Dizdar O, Babacan T, Sahin I, Ararat E, et al. Outcomes of locally advanced breast cancer patients with ≥ 10 positive axillary lymph nodes. Med Oncol 2013;30:615.

4. Kim JM, Kim JY, Jung EJ, Kwag SJ, Park JH, Park T, et al. The prognosis factors among breast cancer patients with extensive axillary lymph node metastasis. Korean J Clin Oncol 2018;14:43-7.

7. Liao Y, Yin G, Fan X. The positive lymph node ratio predicts survival in T1− 4N1− 3M0 non-small cell lung cancer: a nomogram using the SEER database. Front Oncol 2020;10:1356.


8. Cetin IA, Akay SU, Ozkok HBC, Sengoz M. Lymph node ratio as an independent prognostic factor for breast cancer-related mortality in patients with node-positive breast cancer. J Cancer Res Ther 2020;16:1387.

11. Noh JM, Kim KH, Park W, Suh CO, Huh SJ, Choi DH, et al. Prognostic significance of nodal involvement region in clinical stage IIIc breast cancer patients who received primary systemic treatment, surgery, and radiotherapy. Breast 2015;24:637-41.


12. Spronk PER, van Bommel, Siesling S, Wouters M, Peeters MV, Smorenburg CH. Variation in use of neoadjuvant chemotherapy in patients with stage III breast cancer: results of the Dutch national breast cancer audit. Breast 2017;36:34-8.


13. Ai X, Liao X, Li J, Tang P, Jiang J. Clinical outcomes of N3 breast cancer: a real-world study of a single institution and the US surveillance, epidemiology, and end results (SEER) database. Cancer Manag Res 2020;12:5331.


15. Grassadonia A, Vici P, Gamucci T, Moscetti L, Pizzuti L, Mentuccia L, et al. Long-term outcome of breast cancer patients with pathologic N3a lymph node stage. Breast 2017;32:79-86.


16. Liu D, Chen Y, Deng M, Xie G, Wang J, Zhang L, et al. Lymph node ratio and breast cancer prognosis: a meta-analysis. Breast Cancer 2014;21:1-9.


20. De la, Chambergo‐Michilot D, Valcarcel B, Rebaza P, Möller M, Araujo JM, et al. Lymph node ratio as best prognostic factor in triple‐negative breast cancer patients with residual disease after neoadjuvant chemotherapy. Breast J 2020;26:1659-66.


22. Jin ML, Gong Y, Pei YC, Ji P, Hu X, Shao ZM. Modified lymph node ratio improves the prognostic predictive ability for breast cancer patients compared with other lymph node staging systems. Breast 2020;49:93-100.


23. Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 2007;13:4429-34.


26. Luo SP, Wu QS, Chen H, Wang XX, Chen QX, Zhang J, et al. Validation of the prognostic significance of the prognostic stage group according to the eighth edition of American Cancer Joint Committee on Cancer Staging System in triple-negative breast cancer: an analysis from surveillance, epidemiology, and end results 18 database. J Surg Res 2020;247:211-9.


27. Fidalgo F, Rodrigues TC, Pinilla M, Silva AG, Maciel Mdo, Rosenberg C, et al. Lymphovascular invasion and histologic grade are associated with specific genomic profiles in invasive carcinomas of the breast. Tumor Biol 2015;36:1835-48.

28. Schwartz AM, Henson DE, Chen D, Rajamarthandan S. Histologic grade remains a prognostic factor for breast cancer regardless of the number of positive lymph nodes and tumor size: a study of 161 708 cases of breast cancer from the SEER program. Arch Pathol Lab Med 2014;138:1048-52.

29. Perou CM, Sorlie T, Eisen MB, van de, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature 2000;406:747-52.