Predictors of Positive or Close Surgical Margins in Breast-Conserving Surgery for Patients with Breast Cancer
Article information
Abstract
Purpose
This study aimed to determine the clinical and pathological factors associated with a higher rate of positive or close margins after breast-conserving surgery (BCS) by comparing these patients to patients with a negative margin. The second aim was to evaluate intraoperative resection margin status and reoperation rates for margin control in patients who underwent BCS.
Methods
We reviewed the clinical and pathological data of all women diagnosed with invasive breast carcinoma (IBC) and ductal carcinoma in situ (DCIS) at our institution between January 2006 and December 2016.
Results
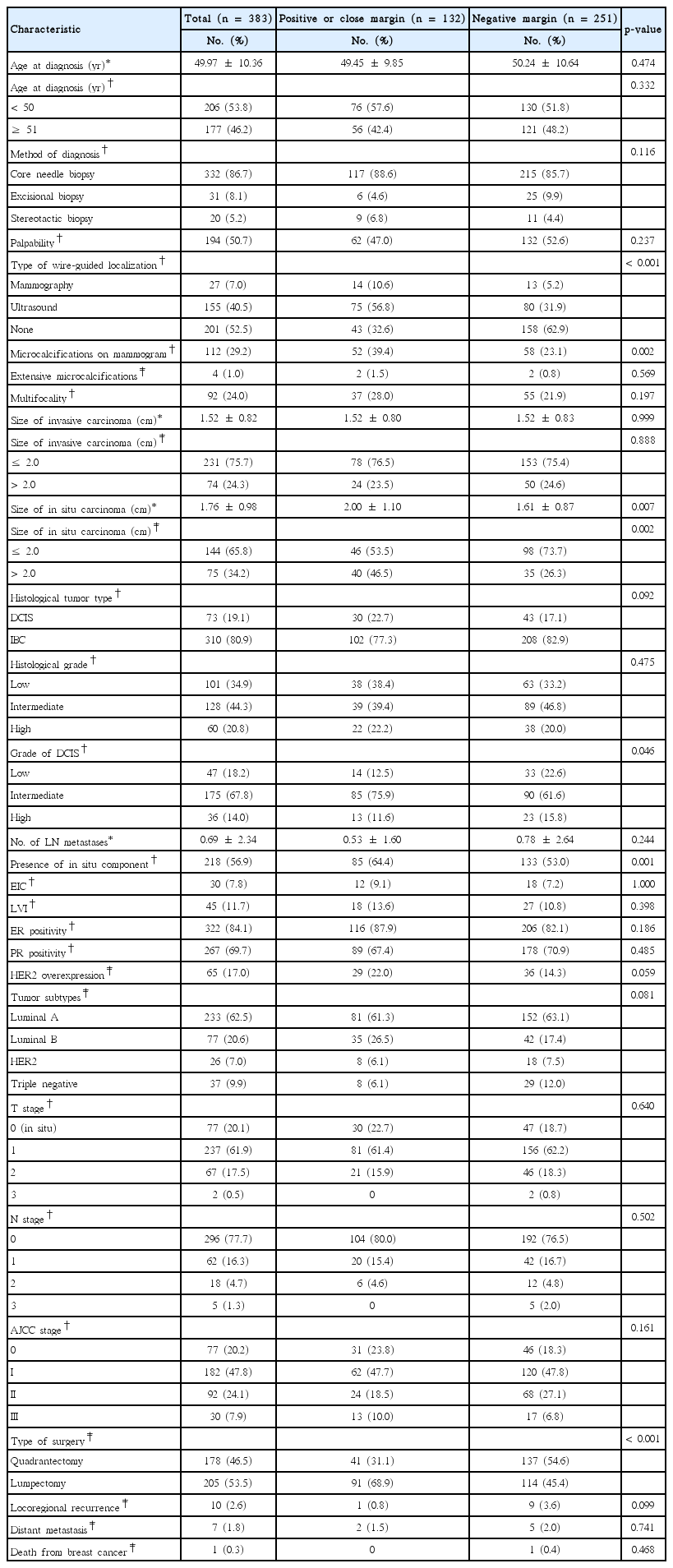
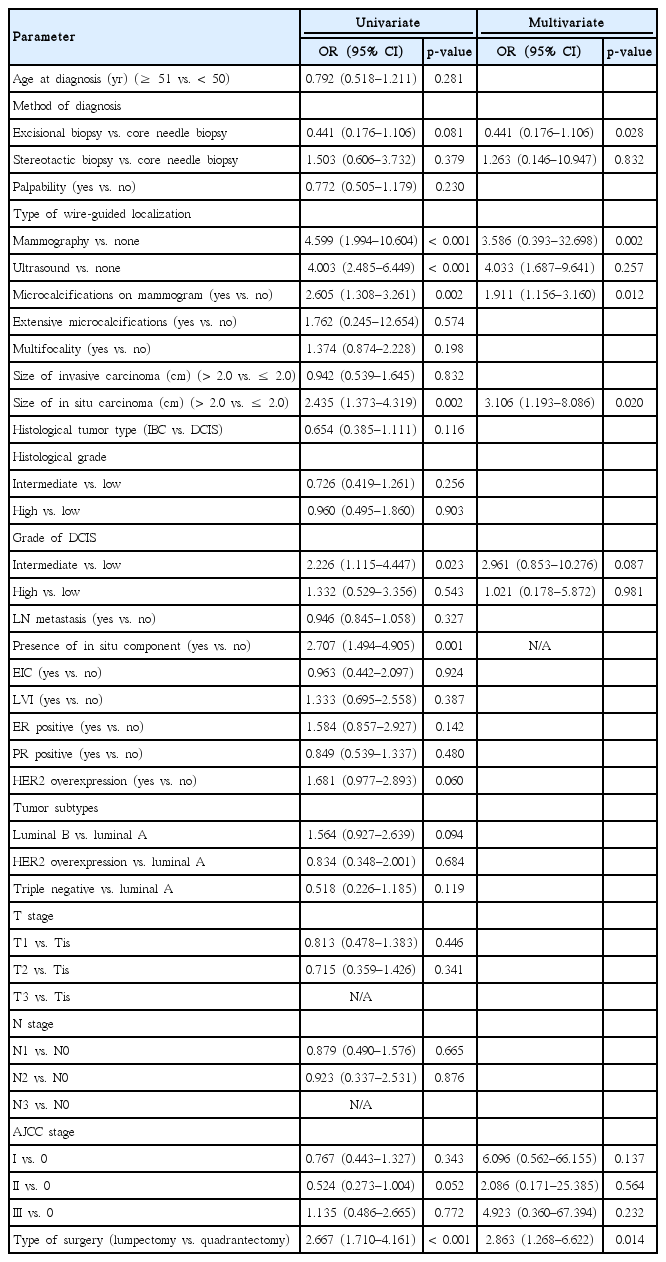
During the 10-year study period, 785 patients were diagnosed with either IBC or DCIS, and 402 of these patients had undergone a total mastectomy as the primary treatment. The remaining 383 patients who underwent BCS were included in the final analysis. Of these, 100 patients (26.1%) had intraoperative positive or close margins. The remaining 283 patients (73.9%) had a negative margin intraoperatively, but 32 of these patients had positive or close margins on permanent sections. In the multivariate analyses, microcalcifications on mammograms (vs. none; odds ratio [OR], 1.911; 95% confidence interval [CI], 1.156–3.160), in situ carcinomas larger than 2.0 cm (vs. ≤2.0 cm; OR, 3.106; 95% CI, 1.193–8.086), and lumpectomy (vs. quadrantectomy; OR, 2.863; 95% CI, 1.268–6.622) showed a significant association with a positive or close surgical margins. Patients with intraoperative positive or close margins underwent more reoperation than those with negative margins (5.0% vs. 2.8%).
Conclusion
After BCS, microcalcifications on mammograms, large-sized in situ carcinomas, and lumpectomy were more likely to have positive or close margins.
INTRODUCTION
Breast cancer is the most common malignancy in women and is the leading cause of death in Western industrialized countries [1]. Since the introduction of mammographic screening in the 1980s, the detection of nonpalpable, small-sized breast cancer has increased. The proportion of patients undergoing mastectomy has decreased, and now more patients are being treated with breast-conserving surgery (BCS) followed by breast irradiation. A randomized trial conducted 20 years of follow-up study to compare the efficacy and survival benefit of radical mastectomy to that of BCS [2]. The long-term survival rate of women undergoing BCS was equivalent to that of women undergoing radical mastectomy. Since these studies, BCS had become a standard treatment for women with relatively small-sized breast cancers as it offers better cosmetic results and reduces the risk of infection.
Despite its cosmetic benefit and efficacy, a major drawback of BCS is the incidence of locoregional recurrence (LRR), even when combined with radiotherapy [3]. Ipsilateral breast tumor recurrence rate is estimated to be 5%–20% over 5–10-years’ follow-up [4]. The patient’s age, tumor size, positive lymph nodes, extensive intraductal component (EIC), lack of adjuvant systemic therapy, and dose and timing of radiotherapy are independent predictors of LRR. Among these factors, the pathologic margin status is the most important predictor [5,6].
However, the definition of an adequate margin is still controversial. Some authors define negative margins as those being more than 1 mm [7], while others advocate margins of 10 mm or more [8]. Recurrence risk has been demonstrated to decrease proportionately with margin width, with the lowest recurrence rates in patients in whom a 10-mm margin is achieved and the highest rates in patients with margins of less than 1 mm [9]. The National Comprehensive Cancer Network (NCCN) guideline and the 2014 Society of Surgical Oncology-American Society for Radiation Oncology Consensus Guidelines on Margins defines a negative margin in infiltrating carcinomas as having “no ink on the tumor” [10,11]. Cases with positive margins should undergo further surgery, such as re-excision or mastectomy. In select cases of microscopically focally positive margins in the absence of an EIC, the use of a higher radiation boost dose to the tumor bed could be considered. The definition of negative margins for ductal carcinoma in situ (DCIS) is somewhat different. The NCCN recommends that margins less than 1 mm are inadequate, and the American Society of Clinical Oncology guideline recommends a 2-mm margin as the standard for DCIS [12].
The key aim of this study was to determine the clinical and pathological factors associated with a higher rate of positive or close margins after BCS by comparing with a group of patients in whom a negative margin was achieved. The second aim was to evaluate the intraoperative resection margin status and the ratio of reoperation for margin control in patients who underwent BCS at our institution.
METHODS
Patient selection
This retrospective cohort study was conducted at the Department of Surgery, Kangbuk Samsung Hospital, and included all patients treated with a diagnosis of breast cancer between January 2006 and December 2016. Patients were selected by reviewing electronic medical charts for invasive breast carcinoma (IBC) and DCIS cases. From this population, only patients undergoing BCS as their first operation were included in the analysis.
Surgery
Two types of BCS were performed: (1) lumpectomy and (2) wide excision. The wide excision group consisted of either quadrantectomy or lumpectomy, with the superficial or deep margins of the excision extending up to the skin and pectoralis fascia, respectively (in this type of extended surgery, no re-excision was required if close or involved margins were superficial or deep). All nonpalpable lesions were wire-guided using ultrasound or mammography, and specimen radiography was performed to confirm the removal of microcalcifications. Specimens were marked with orienting sutures and sent to the pathology department for intraoperative evaluation of margin status.
Pathologic and clinical evaluation
Margin status was classified as positive when invasive or in situ disease were observed at the inked surgical margin, focally positive when the tumor focus was at the inked margin, close when tumor cells were ≤2 mm from the inked margin, and negative when tumor cells were >2 mm from the inked margin [11]. When tumor cells were positive or close to the margin, the tumor was designated for re-excision. When the result was positive or close to the margin after three consecutive margin excisions, BCS was converted to mastectomy. Permanent sections were analyzed using paraffin-embedded blocks.
Patients were characterized based on their clinical characteristics: age at diagnosis; diagnostic method (i.e., core needle biopsy, excisional biopsy, stereotactic biopsy); palpability of the lesion; methods used for tumor localization (i.e., ultrasound, mammography, skin marking); presence of malignancy-related calcification; extensive microcalcifications; multifocality of the lesion; type of surgery (i.e., quadrantectomy, lumpectomy); locoregional recurrence; and distant metastasis and death from breast cancer. Patients were also characterized based on their pathological characteristics: histologic type; size of invasive or in situ carcinoma; grade of DCIS; tumor grade (Scarff-Bloom-Richardson grading modified by Elston and Ellis); number of metastatic lymph nodes; presence of in situ component; EIC; lymphovascular invasion; estrogen receptor (ER) status; progesterone receptor (PR) status; human epidermal growth factor receptor 2 (HER2) status; subtypes; 7th edition of the American Joint Committee on Cancer TNM stage; status of margins; and re-excision or secondary operation. The immunohistochemistry-defined subtypes were as follows: luminal A, ER(+) or PR(+), HER2(–); luminal B, ER(+) or PR(+), HER2(+); HER2-enriched, ER(–) and PR(–), HER2(+); and triple-negative, ER(–) and PR(–), HER2(–) [13]. We further subtyped hormone receptor-positive tumors using the Ki-67 labeling index (<14% for luminal A vs. ≥14% for luminal B) to distinguish luminal B from luminal A tumors [14]. This study was approved by the institutional review boards (KBSMC 2014-10-008). Informed consent was obtained from all patients.
Statistical analysis
Student t-test for continuous variables and Pearson chi-square test for categorical variables were used to evaluate the associations between margin status and clinical and pathological characteristics. Univariate and multivariable logistic regression were used to calculate odds ratios (ORs) with 95% confidence intervals (CIs) for positive or close margins. A p-value <0.05 (two-tailed) was considered statistically significant. All statistical analyses were performed using PASW Statistics for Windows, version 18.0 (SPSS Inc., Chicago, USA).
RESULTS
During the 10-year study period, 785 patients were diagnosed with either IBC or DCIS, and 402 of these patients had undergone a total mastectomy as the primary treatment. As a result, the remaining 383 patients who underwent BCS were included in the final analysis. Of these, 100 patients (26.1%) had intraoperative positive or close margins. The remaining 283 patients (73.9%) had a negative margin intraoperatively, but 32 of these patients had positive or close margins at permanent section. As a result, the positive or close margin group comprised 132 patients, and the negative margin group comprised 251 patients. During the study period, 310 IBC and 73 DCIS patients were evaluated. Among the 310 IBC patients, 284 were diagnosed with invasive ductal carcinoma, eight with invasive lobular carcinoma, and 18 with other types of carcinoma (i.e., tubular, mucinous, and micropapillary). The mean age was 49.9±10.3 years (range, 20–85 years), and the mean sizes of the IBC and DCIS tumors were 1.52 cm and 1.76 cm, respectively.
Comparison of clinicopathological characteristics between the positive or close margin and negative margin groups
Patients who had positive or close margins were more likely to present with microcalcifications on their mammograms (39.4% vs. 23.1%, p=0.002), large-sized in situ carcinomas (2.00 cm vs. 1.61 cm, p=0.007), and an in-situ component accompanied by IBC (64.4% vs. 53.0%, p= 0.001). They were also more likely to undergo lumpectomy (68.9% vs. 45.4%, p<0.001) than patients with a negative margin. A summary of clinical and pathological features is provided in Table 1.
Univariate and multivariate analyses of predictors for a positive or close surgical margin
In the univariate analysis, patients with wire-guided localization using mammography (vs. none; OR, 4.599; 95% CI, 1.994–10.604), ultrasound (vs. none; OR, 4.003; 95% CI, 2.485–6.449), microcalcifications on mammograms (vs. none; OR, 2.605; 95% CI, 1.308–3.261), intermediate grade DCIS (vs. low; OR, 2.226; 95% CI, 1.115–4.447), in situ carcinomas larger than 2.0 cm (vs. ≤2.0 cm; OR, 2.435; 95% CI, 1.373–4.319), the presence of an in situ component (vs. none; OR, 2.707; 95% CI, 1.494–4.905), and lumpectomy (vs. quadrantectomy; OR, 2.667; 95% CI, 1.710–4.161) showed a significant association with a positive or close surgical margin (Table 2).
In the multivariate analysis, microcalcifications on mammograms (vs. none; OR, 1.911; 95% CI, 1.156–3.160), in situ carcinomas larger than 2.0 cm (vs. ≤2.0 cm; OR, 3.106; 95% CI, 1.193–8.086), and lum-pectomy (vs. quadrantectomy; OR, 2.863; 95% CI, 1.268–6.622) showed a significant association with a positive or close surgical margin (Table 2).
Intraoperative margin assessment and decisions regarding re-excision
The results of the intraoperative margin assessment, re-excision rate analysis, and final margin assessment are shown in Figure 1. While 100 patients (26.1%) were found to have positive or close margins on intraoperative evaluation, 88 of them underwent re-excision of the involved margin during the same operation. All except five patients achieved a final negative margin. These remaining five patients had three consecutive positive or close margins, and a total mastectomy was ultimately performed. Twelve patients with intraoperative positive or close margins did not undergo re-excision but instead planned to be re-evaluated during the permanent section analysis. Permanent margin status was positive for all 12 patients. Only five of the 12 patients underwent a secondary operation (total mastectomy, 2; margin re-excision, 3). The remaining seven patients did not undergo re-excision because the directions of the positive or close margins were superficial or deep so that there was no remaining breast tissue. These patients were treated with boost radiation therapy, and no recurrences were reported during the 10-year follow-up period. Among 100 patients with intraoperative positive or close margins, five (5.0%) underwent secondary operations. Among 283 patients with intraoperative negative margins, eight (2.8%) underwent secondary operations. Patients with intraoperative positive or close margins underwent more reoperation than those with negative margins (5.0% vs. 2.8%).
Final margin assessment and decisions regarding re-excision
Thirty-two patients (8.3%) had negative margins on intraoperative assessment but positive margins on permanent pathologic evaluation. Among this population, eight patients underwent a secondary operation (total mastectomy, 5; margin re-excision, 3). The remaining 24 patients did not undergo a secondary operation because the direction of the positive or close margins was superficial or deep so that there was no remaining breast tissue. These patients were treated with boost radiation therapy, and no recurrences were reported during the 10-year follow-up period.
DISCUSSION
After BCS, the LRR rate ranges from 5% to 20% after 5–10 years [4]. Many risk factors of LRR are known, such as the age at diagnosis, tumor size, lymph node metastasis, EIC, adjuvant systemic therapy, the amount and initiation date of radiation therapy, and the free margin status during operation. The status of the surgical margins is the most important factor, as it directly affects the risk of recurrence [5,6]. Since the National Surgical Adjuvant Breast and Bowel Project first introduced the importance of margin status in 1989, the rate of local recurrence has decreased, and the overall survival rate for BCS has become comparable to that of mastectomy [15]. However, there is currently no clear indication for positive or close margins. The definition of a positive margin varies, such that cancer cells can be from 1 to 5 mm from the margin [7,16].
Our findings revealed that wire-guided localization using mammography and ultrasound, microcalcifications on mammograms, an intermediate grade of DCIS, large-sized in situ carcinomas, the presence of an in-situ component accompanied by IBC, and lumpectomy were associated with positive or close margins after BCS. Several researchers have reported predictors of margin positivity and re-excision [17]. Tumor size, nodal positivity, multifocality, EIC, lobular histology, and young age were often regarded as significant factors [16].
In our study, we found that the likelihood of a positive or close margin was increased for large-sized (>2.0 cm) in situ carcinomas. In situ components accompanied by IBC and microcalcifications on mammograms also correlated with positive or close margin status in univariate analysis (Table 2). Based on these results, it is essential to detect the presence of in situ components at the surgical margin during intraoperative frozen biopsies. Intraoperative specimen mammography is also essential for confirming the retrieval of microcalcifications and thereby reducing the chance of positive margins. However, the margin of DCIS is not well defined, and the extension into breast tissue is often difficult to determine, which results in a high rate of reoperation [18]. The rate of reoperation owing to positive margins was twice as high when tumors had an in situ component [19]. The presence of microcalcifications is another factor that makes it difficult to interpret margin status. Therefore, patients with microcalcifications on preoperative mammograms should be identified for margin positivity and secondary operation.
Wire-guided localization using either mammography or ultrasound is the standard technique for locating tumors before surgery for nonpalpable breast cancers. However, it is more difficult to achieve negative margins in nonpalpable lesions due to technical factors and the diversity in its radiographic appearance [20]. The wire can be displaced, leading to an inaccurate localization, or it can be transected during surgery [21]. For these reasons, we also found a likelihood of positive or close margins with ORs of 4.599 and 4.003 for wire-guided localization using mammography and ultrasound, respectively. A recent study reported intraoperative wire-guided localization using ultrasound in BCS to decrease positive margin status and improve assessment of margin status [22]. Further studies are needed to assess the validity of wire-guided localization in terms of positive or close margins.
There are two types of BCS. It is assumed that wide excision may be superior in terms of local radicality, and lumpectomy may be better in terms of cosmetic outcome [23]. Currently, there are few studies comparing quadrantectomy and lumpectomy regarding disease control outcomes, such as LRR or distant metastasis. However, some have reported no significant difference in ipsilateral breast tumor recurrence between quadrantectomy and lumpectomy if adequate surgical margins could be achieved [23]. Quadrantectomy removes the entire quadrant of the breast containing the primary tumor with overlying skin and the fascia of the major pectoralis muscle and was first described by Veronesi et al. [24]. Lumpectomy completely excises the lesion with at least 1 cm of tissue around the clinical margin of the tumor [25]. We found that lumpectomy, compared to wide excision, increased the likelihood of positive or close margins. Consistent with our result, Ramanah et al. [26] also showed that quadrantectomy decreased the rate of re-excision. However, others found that when close or involved margins were superficial and deep, re-excision showed no residual tumor [27]. Therefore, further discussions are necessary to determine the appropriate surgery to decrease positive or close margins. Surgeons also need to explain to patients with large-size (>2.0 cm) in situ carcinoma who are initially scheduled to undergo lumpectomy that the possibility of resecting larger margins than planned and converting to wide excision exists.
To avoid positive or close margin status after BCS, a high accuracy of intraoperative frozen section analysis (FSA) is required. Freezing tissues in a glycol-based polyethylene-embedding compound can be performed in a relatively short period intraoperatively, yielding an accuracy of 97% with sensitivity and specificity rates between 95% and 99% [28]. However, the relatively high false negative rate of FSA or the number of two-stage procedures, due to discrepancies between FSA and the final pathological report, reportedly ranges from 0% to 19% [29]. New methods for intraoperative margin assessment, such as hand-held positron emission tomography probes or the radio-guided occult lesion localization method, have been introduced to overcome this limitation [30].
In conclusion, we have confirmed that wire-guided localization using mammography and ultrasound, the presence of microcalcifications on mammograms, large-sized in situ carcinomas, the presence of an in-situ component accompanied by IBC, and lumpectomy increased the likelihood of positive or close margins after BCS. Patients with these factors should be considered for and advised about the need for further surgery. A multidisciplinary team approach should also be applied to decrease the chance of positive or close margins and achieve effective local control.
Notes
The authors declare that they have no competing interests.