Axillary Lymph Node to Primary Breast Tumor Standardized Uptake Value Ratio from FDG-PET/CT Imaging for Predicting the Necessity for Nodal Dissection in Primary Breast Tumors
Article information
Abstract
Purpose
Accurate preoperative detection by radiologic assessment is necessary to specifically identify patients with at least three positive nodes, who can directly undergo axillary lymph node (ALN) dissection, and avoid unnecessary surgical procedures. We evaluated the usefulness of the standardized uptake value (SUV) ratio of ALN in primary breast tumor, using 18F-fluorodeoxyglucose positron emission tomography (FDG-PET)/computed tomography (CT) and magnetic resonance imaging (MRI) to predict the necessity of ALN dissection during breast cancer surgery.
Methods
In this retrospective study we enrolled 316 consecutive patients with invasive breast cancer. The SUV ratio of ALN to primary breast tumor uptake was calculated. Optimal cutoff values were determined by receiver operating characteristic curve analysis for predicting the presence of ≥3 ALN metastases. Diagnostic performance of FDG-PET and MRI features for the prediction of ≥3 ALN metastases were determined by sensitivity, specificity, and diagnostic odds ratio (DOR). A subgroup analysis for FDG-avid tumors was also performed.
Results
Of the 316 patients, 36 (11.4%) showed involvement of ≥3 ALNs, with 101 (32%) having at least one metastatic lymph node. Axillary 18F-FDG uptake was positive in 75 patients (23.7%), and the optimal ratio of maximum SUV of axillary lymph node and primary tumor for determining ALN dissection was 0.3. MRI scans revealed suspicious ALN involvement in 147 patients (46.6%). The sensitivity and specificity of MRI detection were 88.9% and 56.2%, respectively, while for SUVLN/T ratio, they were 69.4% and 86.8%, respectively. DOR values for MRI and SUVLN/T ratio were 10.37 and 9.7, respectively. The area under the curve (AUC) was improved to 0.896 (95% confidence interval [CI], 0.817–0.975) for the SUVLN/T ratio in patients with FDG-avid primary tumors (FDG ≥3.9, n=108), but the MRI AUC was worsened (0.681; 95% CI, 0.569–0.793). The DOR, sensitivity, and specificity for the SUVLN/T ratio of FDG-avid cancers were 25.68, 89.0% and 86.0%, respectively.
Conclusion
SUVLN/T ratio outperformed MRI features in predicting the need for ALN dissection in FDG-avid primary breast cancer. PET/CT may be a potential noninvasive diagnostic technique for identifying the presence of ≥3 ALN metastases.
INTRODUCTION
The American College of Surgeons Oncology Group Z0011 trial indicated that complete axillary lymph node dissection (ALND) did not improve survival in women with clinical T1-T2 tumors and 1 to 2 involved axillary nodes, who underwent lumpectomy with radiation therapy, followed by systemic therapy [1]. As the treatment for both the axillary lymph nodes (ALNs) and primary breast tumors evolve, predicting the status of the ALNs has become increasingly important. However, intraoperative frozen sections of sentinel lymph node (SLNs) or imaging-guided ALN biopsy still show a high false-negative rate of approximately 14% to 43% [2,3].
To appropriately determine the necessity of ALND, evaluation of the axillary tumor burden is critical. Ultrasonography, computed tomography (CT), or magnetic resonance imaging (MRI) primarily depend on lymph node (LN) size or morphology for differentiation between metastatic and nonmetastatic status. Therefore, normal- and small-size metastatic LNs can easily remain undetected. Furthermore, the variable morphologic characteristics of LNs also reduce diagnostic accuracy owing to the overlapping features of metastatic and nonmetastatic LNs [4].
Following the advances in preoperative imaging techniques, some investigators have described the usefulness of 18F-fluorodeoxyglucose positron emission tomography (FDG-PET)/CT, a metabolic imaging modality, for ALN staging in breast cancer [5,6]. However, the reported sensitivity and specificity of FDG-PET/CT for ALN metastasis are low at 48% to 58% and 84% to 95%, respectively [7,8].
We hypothesized that, although it would be difficult to distinguish involvement of a single LN, it may be possible to use FDG-PET/CT to predict high LN tumor burden, defined as involvement of 3 ALNs. In the present study, we evaluated the usefulness of the standardized uptake value (SUV) ratio of ALN to primary breast tumor, as determined by FDG-PET/CT, as compared to MRI, for predicting the necessity of ALND in breast cancer surgery.
METHODS
Study design
The electronic medical records of 429 consecutive patients with invasive breast cancer, treated at Chung-Ang University Hospital between January 2012 and December 2016, were retrospectively analyzed. Patients were included if they (1) had histopathologically confirmed invasive breast cancer and (2) undergone pretreatment breast MRI and FDG-PET/CT imaging. Patients were excluded according to the following criteria: (1) any prior treatment for primary breast cancer; (2) ductal carcinoma in situ; or (3) breast cancer type other than adenocarcinoma. Finally, 316 consecutive patients were enrolled in the study following application of the inclusion/exclusion criteria. Patients underwent ALND if there was at least one metastatic sentinel LN from the frozen biopsy result collected intraoperatively. This study was reviewed and approved by the Institutional Review Board of Chung-Ang University Hospital (IRB number: 1711-011-16120), and the informed consent was waived.
Radiologic analyses
PET/CT scans were performed on a Biograph mCT/128 PET/CT scanner (Siemens Medical Solutions, Hoffman Estates, USA). All
patients fasted for at least 6 hours before being administered FDG (4.81 MBq/kg of body weight, intravenously) and had blood glucose levels less than 150 mg/dL. Whole-body PET/CT was performed from the skull base to the proximal thigh in the supine position. PET/CT imaging began 60 minutes after FDG injection. Ellipsoid volumes of interest that included the entire breast tumor and ALNs were segmented, and maximum SUVs of the breast tumor (SUVT) and ALNs (SUVLN) were measured on each PET/CT data set. SUVLN to SUVT ratios (SUVLN/T) were then calculated.
MRI scans were acquired on a 3.0 Tesla scanner (Achieva; Philips Medical Systems, Best, the Netherlands), using a 7-channel dedicated breast coil system with the patient in the prone position. MRI was performed as reported in a previous study [9].
Evaluation of ALN status was performed using visual analysis on FDG-PET. Positivity was defined as a perceptibly higher metabolism of ALN compared to the normal background soft tissue [10]. MRI scans were defined to be positive for ALN metastasis if the characteristics of LNs included the following: >10 mm in size, rounded shape, eccentric cortical hypertrophy, or abnormal enhancement on T1-
weighted images.
Statistical analyses
Receiver operating characteristic (ROC) analysis was conducted to determine the abilities of the SUVLN/T and breast MRI scans to detect involvement of ≥3 ALNs. The optimal SUVLN/T that offered the highest sum of sensitivity and specificity for detecting ALN metastasis was determined. The sensitivity, specificity, positive predictive value, and negative predictive value of both the SUVLN/T and breast MRI were then calculated. Diagnostic performances were compared using diagnostic odds ratio (DOR) and area under the curve (AUC) from ROC analysis. Statistical analyses were performed using SPSS version 23.0 (IBM Corp., Armonk, USA). Two-tailed p-values <0.05 were considered statistically significant.
In a subgroup of patients who showed FDG-avid primary tumors (divided by the mean SUV of the primary breast tumor), predictability of the SUVLN/T and MRI scan was assessed by ROC curves, sensitivity, specificity, and DOR. The Differences between FDG-avid and nonavid patient groups were evaluated using Student t-test, the chi-square test, or Fisher exact test.
RESULTS
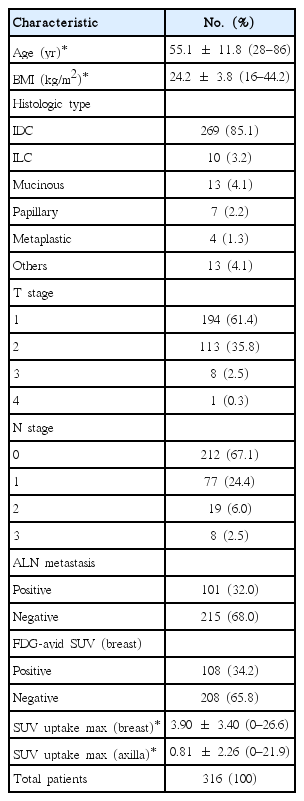
A total of 316 women (mean age, 55.1±11.8 years) who had been diagnosed with breast cancer were included in this study. The demographic characteristics of the patients are presented in Table 1.
The histology for 85% of patients was invasive ductal carcinoma. ALN metastases were histologically confirmed by ALND or SLN biopsy. The flow chart of axillary treatment in our institute is shown in Figure 1. Of the 316 study patients, 36 (11.4%) showed involvement of 3 ALNs, and 101 (32%) had at least one metastatic LN.

Flow chart of axillary treatment.
NCT=neoadjuvant chemotherapy; DCIS=ductal carcinoma in situ; US=ultrasonography; ALN=axillary lymph node; SLNB=sentinel lymph node biopsy; CNB=core needle biopsy; ALND=axillary lymph node dissection.
Axillary FDG uptake was positive in 75 (23.7%) of the study patients. The mean SUV of the primary tumor was 3.9 (range, 0.0–26.6), while the mean SUV of the ALNs was 0.81 (range, 0.0–21.9). In 23 patients (7.3%), the primary tumor showed no FDG uptake. Among these patients, 21 demonstrated no FDG uptake, but two patients showed FDG uptake only in ALNs. We excluded the SUVLN/T results of these 23 patients because they are incalculable. Based on ROC curve analysis, an optimal SUVLN/T cutoff point of 0.3 was determined for predicting the necessity of ALND(3 ALN metastases).
Suspicious ALN involvement was observed on MRI in 147 patients (46.6%). The sensitivity and specificity of MRI for predicting the necessity of ALND were 88.9% and 56.2%, respectively, while those of SUVLN/T were 69.4% and 86.8%. AUCs for MRI scans and SUVLN/T were 0.756 (95% confidence interval [CI], 0.682–0.829) and 0.817 (95% CI, 0.733–0.900), respectively. Further analysis of DOR determined values of 10.37 and 9.7 for MRI scans and SUVLN/T, respectively.
However, in a subgroup of patients with FDG-avid primary tumors (FDG uptake >3.9, n=108), the AUC improved to 0.896 (95% CI, 0.817–0.975) for SUVLN/T, while the AUC of MRI scans decreased to 0.681 (95% CI, 0.569–0.793). The DOR, sensitivity, and specificity of the SUVLN/T for FDG-avid tumors was 25.68, 89.0% and 86.0%, respectively (Figure 2). Based on ROC curve analysis, the optimal cutoff point of SUVLN/T was 0.27 for predicting the necessity of ALND(3 ALN metastases). Diagnostic performance of the SUVLN/T ratio and MRI are shown in Table 2.
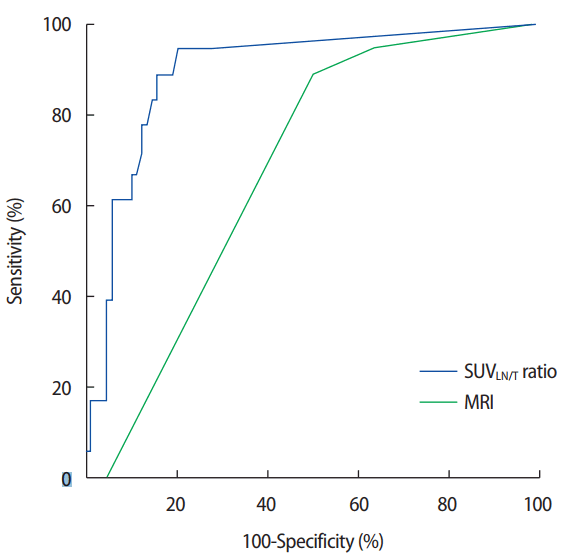
Receiver operating characteristic curves for SUVLN/T ratio in a subgroup with FDG-avid breast tumors.
SUVLN/T ratio=ratio of maximum standardized uptake value (SUV) of axillary lymph node and primary tumor; MRI=magnetic resonance imaging; FDG=18F-fluorodeoxyglucose.
We also compared FDG avidity and certain patient characteristics, which as shown in Table 3. A higher SUV for primary breast tumors correlated with multiple prognostic variables, including tumor size, estrogen receptor (ER) negativity, and progesterone receptor (PR) negativity.
DISCUSSION
The aim of the present study was to assess the predictive value of the LN to tumor SUV ratio in patients with breast cancer. We compared MRI scans and FDG-uptake, from PET/CT imaging, for the detection of three or more axillary metastases. Our study showed that the SUVLN/T could accurately predict the necessity of ALND dissection for patients with FDG-avid primary breast cancer.
Advances in preoperative imaging have advanced and optimized axillary management [11]. For minimally invasive breast cancer surgery, preoperative evaluation of the ALNs is important. There have been a few reported studies comparing MRI and PET/CT for the diagnosis of LN metastasis. Recently, Liang et al. [12] reported a meta-analysis that suggested MRI scans had higher sensitivity than PET/CT imaging (0.82 vs. 0.64) for the diagnosis of ALN metastasis in patients with breast cancer.
For distinguishing the involvement of at least one LN, PET parameters showed limited diagnostic performance, attributed to the partial volume effect, relatively low FDG-uptake by low-grade malignancies, and FDG-uptake by benign entities [13,14]. Nonetheless, FDG-PET provides metabolic information, and hence, combination of the FDG-PET with other imaging modalities may improve the diagnostic accuracy for detecting ALN metastasis in breast cancer [15]. In addition, SUVLN/T might be a more objective diagnostic method than axillary ultrasonography, which is operator-dependent, for predicting LN metastasis.
Previous studies showed that the accuracy of SUVLN/T for predicting the presence of ALN metastasis was superior to that of SUVLN [15]. This finding may result from variation in SUV measurements from multiple hospital protocols, as well as the influence of factors including body composition, blood glucose levels, length of the uptake period, and the partial volume effect. In addition, SUVLN is related to the FDG avidity of the primary tumor [16,17]. Therefore, the axillary SUVLN/T might predict LN status more objectively than nodal SUV alone.
An important finding of the present study is that SUVLN/T appears to be particularly reliable in patients with an SUVT greater than 3.9. Therefore, tumors with a low SUV, including low-grade malignancies or tumors combined with benign inflammatory lesions, warrant additional morphological evaluation by conventional modalities. Higher SUV for primary breast tumors in our study correlated with tumor size, ER negativity, and PR negativity. Furthermore, Peterson et al. [18] and Gemignani et al. [19] found an association with body mass index, sex hormone-binding globulin, and PR expression in 18F-FES PET uptake.
In the present study, the specificity for predicting three or more ALN metastases using SUVLN/T was much higher than the specificity achieved using MRI. To achieve a higher specificity, SLN biopsy might be an optional but nonessential procedure in patients with highly suspicious ALN metastasis, as determined by PET/CT imaging; these patients could undergo complete ALND as the primary procedure, an approach that would reduce time and cost.
The limitations of the present study include the possibility of inherent biases associated with a retrospective study design, as well as the small sample size. In addition, PET/CT scans were interpreted by a single, albeit experienced, nuclear medicine physician.
Axillary LN evaluation is crucial for staging and therapeutic planning in patients with invasive breast cancer. Although the current data were retrospectively analyzed at a single institution, our findings suggest that SUVLN/T, as obtained by PET/CT, may help predict the presence of three or more ALN metastases in an FDG-avid subgroup prior to surgery. A large, prospective cohort study may be recommended to validate SUVLN/T as a reliable predictor of three or more metastases in the ALNs.
In conclusion, SUVLN/T can identify the presence of three or more ALN metastases, particularly in patients with FDG-avid breast tumors. PET/CT imaging may be a valuable tool for treatment selection in advanced breast cancer.
Notes
The authors declare that they have no competing interests.