Usefulness of Fine-Needle Aspiration Biopsy before Performing Ultrasound-Guided Vacuum-Assisted Excision
Article information
Abstract
Purpose:
Ultrasound-guided vacuum-assisted excision (US-VAE) is considered the less invasive method for the histological assessment of breast lesions than surgical excision and also used for removing benign lesions. Some benign lesions require further excision after removing them by US-VAE, because pathologic diagnosis with specimen obtained by US-VAE cannot be confirmative. However, a well-targeted fineneedle aspiration biopsy (FNAB) before US-VAE can provide preoperative diagnosis. The aim of this study is to evaluate the accuracy and safety of FNAB to minimize further excision after performing US-VAE.
Methods:
From June 2007 to December 2009, US-VAE was performed on 321 patients with benign breast lesions which diagnosed by FNAB. Clinicopathological data, medical records and imaging studies were reviewed. We estimated the further excision rate after carrying out US-VAE and evaluated effectiveness of FNAB for pathologic diagnosis of breast lesions before performing US-VAE.
Results:
Of 321 lesions, 118 (27.1%) were diagnosed as specific benign, 201 patients (57.9%) as other nonspecific benign or negative malignant cell, 2 (0.6%) as atypical ductal hyperplasia (ADH) at FNAB. The pathologic diagnoses after US-VAE were usually specific benign diseases; fibroadenoma (190 cases, 59.2%), fibrocystic change (51 cases, 15.9%), other benign (68 cases, 21.2%). As indeterminate lesions, ADH (5 cases, 1.6%), borderline phyllodes tumor (4 cases, 1.2%) were diagnosed. Of 321, only three patients (0.9%) were underwent further excision for malignancy. They were diagnosed as malignant after taking US-VAE, two lobular carcinoma in situ and one invasive ductal carcinoma.
Conclusion:
US-VAE is relatively accurate and effective for removing benign lesion of breast. To reduce the further excision rate, the cytological and pathological confirmation using FNAB should be performed precisely before performing the US-VAE.
INTRODUCTION
Ultrasound-guided vacuum-assisted excision (US-VAE) is an alternative to surgical option for management of breast lesions and can substitute for surgical excision [1,2]. Advantages of this procedure include the followings: in case of typical benign breast diseases, perioperative risks and scarring can be avoided or minimized. In case of with preoperative malignant diagnosis, treatment or surgical procedure can be better planned. Prior to US-VAE, a preoperative diagnosis is obtained by a combination of clinical examination, imaging, and needle biopsy. Clinical diagnoses alone are often unreliable and cannot exclude a possibility of malignancy. The most appropriate means for the preoperative diagnosis is image-guided core needle biopsy (CNB) or fine-needle aspiration biopsy (FNAB). It is well known that CNB is superior to FNAB, but more invasive procedure than FNAB. It is also well known that well done FNAB is not inferior to CNB [3]. After US-VAE for breast lesion, the lesions are usually divided into benign, malignant, indeterminate lesion. For benign lesions, mammographic or ultrasonographic follow-up in 6 months or 1 year is advised. Malignant entities including ductal carcinoma in situ and invasive carcinomas are referred to further surgical excision. Surgical excision is recommended in cases of indeterminate lesion if incomplete excision was done by USVAE [4,5]. Therefore, if there is a concordance between pathologic diagnosis of FNAB and US-VAE, the patients are advised to return for follow-up in 6 months. On the other hand in case of discordance, complete surgical excision is recommended [1]. A sufficiently accurate method of performing FNAB before US-VAE may allow many patients to avoid further excision after US-VAE. They provide histological diagnosis with a comparable high degree and have been proven to help reduce the number of unnecessary surgeries for benign disease [6]. The aim of this study is to evaluate the accuracy and usefulness of FNAB to minimize further excision after performing US-VAE.
METHODS
Study design and patients
From June 2007 to December 2009, US-VAE were performed on 321 patients with benign breast lesions in our institution and all the patients were enrolled in this study retrospectively. All of the lesions were category 3 and 4 as determined by ultrasound imaging according to the American College of Radiology Breast Imaging Reporting and Data System (BI-RADS) [7] and had been confirmed as benign by a previous FNAB. Patients had undergone US-VAE for the purpose of complete excision of breast tumors. Indications for removal included severe anxiety of a patient with a palpable mass, tenderness or pain, increasing size, change in shape in a suspicious direction, causing serous nipple discharge, discomfort caused by the palpable mass and also patients’ desire to remove.
The following patient data were recorded: past medical history, age, other clinicopathologic data, imaging characteristics of lesion including size and ultrasonographic imaging feature. We estimated the re-excision rate after carrying out US-VAE and evaluated effectiveness of FNAB for pathologic diagnosis of breast lesions before performing US-VAE. All procedures were performed by two surgeons.
Ultrasound-guided fine-needle aspiration procedure
After ultrasonographic identification of the breast lesion with the patient in the supine position and the ipsilateral arm elevated above the head, FNAB was performed. FNAB using a high-resolution 10-14 MHz linear array transducer with adjustable puncture and biopsy guides (Falcon Premium 2101; BK Medical, Herlev, Denmark) was carried out. Patients were prepared and the breast lesions identified. The fine-needle aspiration (FNA) needle was placed in the lesion and specimen was taken with at least 10 passes widely without full needle withdrawal and under constant negative pressure. We called this procedure that needle can reach to all directions within the lesion as ‘Fan technique.’ The cytology was fixed immediately under alcohol in the outpatient clinic to obtain examinable cells.
Technique of US-VAE procedure
We performed a breast ultrasound examination for all lesions before the removal procedure. The procedure was performed by five surgeons, with an 11- or 8-gauge Mammotome Hand-Held (Ethicon Endo-Surgery, Cincinnati, USA) using a freehand US guidance.
The patient was placed in supine position. After 2 to 3 mL of a local anesthetic agent mixed with epinephrine (1:200,000) was injected in the cutaneous layer, an additional anesthetic agent mixed with normal saline was injected around the mass and along the estimated course of the probe for loosening of the breast tissue. For masses abutting the pectoralis muscle or masses just beneath the skin or near the nipple, further injection administered between the structures and masses to artificially increase the distance for needle passage and to increase safety. A small skin incision (3 mm) was made and the probe was positioned into the lesion by US guidance. Probe approach to the lesion from the insertion site was along the long axis of the lesion, parallel to the muscle plane, and peripheral to the central direction in the same quadrant of the breast. And it advanced to just beneath the mass. Repeated samplings with negative pressure were taken until the lesion was completely eliminated on real-time ultrasound imaging. Residual lesions or complications were evaluated again. When evaluation of residual lesions was disturbed by a fluid collection or surrounding edema, squeezing the site with fingers or compression with the ultrasound probe aided in the evaluation. If there was any suspicion of a residual lesion, the procedures were repeated. At the end, hemostasis was achieved by means of manual compression at least for 10 minutes.
Statistical analysis
A computer-aided analysis program, SPSS version 15.0 for Windows (SPSS Inc., Chicago, USA), was used for all data analysis. A Student t-test was employed to evaluate the relation between all clinicopathologic data, radiologic findings and re-excision after performing US-VAE. All the reported univariate p-values were two sided, and a p-value 0.05 or less was considered to be significant. We estimated the re-excision rate among patients undertaken US-VAE and the concordance rate of the pathologic results between FNAB and US-VAE.
RESULTS
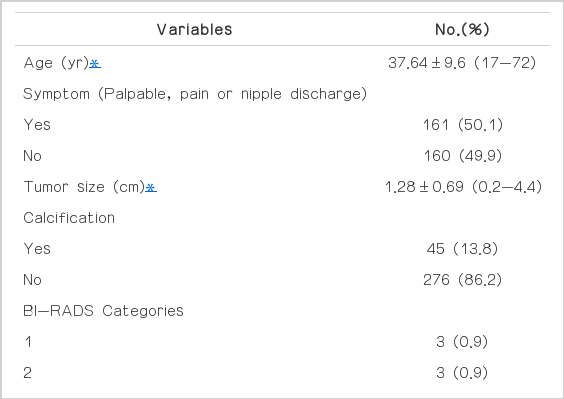
FNAB under US guidance was performed to assess the breast lesions by ascertaining a histological diagnosis before taking US-VAE in all patients. A total of 321 FNAB were performed. Follow-up period was 22.8 months. The mean age of the patients was 37.64±9.6 years ranging between 17 and 72 years old. On US, the mean size of the lesion was 1.28±0.69 cm ranging between 0.2 cm and 4.4 cm. Calcification of tumor on US was showed in 45 patients (13.8%). The lesions were classified by US examination as category 1 in 0.9% of the cases, category 2 in 0.9%, category 3 in 57.6%, category 4A in 34.6%, and category 4B in 4.6%. One hundred sixty-one patients (50.1%) had certain symptom associated breast lesion, of these 161, palpable mass was appeared in 136 (40.9%), pain was 54 (17.6%), and other symptom including nipple discharge was showed in 12 patients (3.5%). Patients’ clin-ical information and tumor characteristics are presented in Table 1.
Of 321 lesions, 118 (27.1%) were diagnosed as specific benign, 201 patients (57.9%) as other nonspecific benign or negative malignant cell, 2 (0.6%) as atypical ductal hyperplasia at FNAB (Table 2). The pathologic diagnoses after US-VAE were usually specific benign diseases; fibroadenoma (190, 59.2%), fibrocystic change (51, 15.9%), other benign (68, 21.2%). As indeterminate lesions, atypical ductal hyperplasia (ADH) (5, 1.6%), borderline phyllodes tumor (4, 1.2%) were diag-nosed (Table 3). Of 321, only three patients (0.9%) were underwent further excision for malignancy. They were diagnosed as malignant after taking US-VAE, two lobular carcinoma in situ and one invasive ductal carcinoma. In all cases of malignancy, surgical excision was followed after taking US-VAE. Intermediate disease, ADH and borderline phyllodes tumor were not followed by surgical excision, because the masses were completely excised and no residual masses were showed on the US after performing US-VAE. The all possible remaining benign lesions were recommended 6 months later follow-up.
All case of malignancy was BI-RADS category 4A, none of all showed calcification on. One patient had certain symptom associated with the breast mass, palpable and nipple discharge. Finally, of 321 patients, only three patients (0.9%) were underwent re-excision after US-VAE. The overall concordance rate of typical histologic diagnosis between FNAB and US-VAE was 82.7% (265/321). The overall accuracy of FNAB in benign lesion was 99% (318/321).
DISCUSSION
Breast cancer is the second most common type of cancer in women with increasing number. However, significantly more women will develop benign breast disease during their adult lives. Although benign breast disease is not typically life threatening, it can cause patient’s discomfort, anxiety, and fear. Not all benign or presumably benign breast masses need to be removed. Removal of breast benign lesion was recommended when patients want to remove the lesions on follow-up periods and for some lesions that are increasing in size or changing in a suspicious direction. Other indications for removal included a palpable mass with tenderness or pain, a palpable mass with a family history of breast cancer, a palpable mass causing several discomforts. For cases that do require removal, surgical excision requires hospital admission, general anesthesia, and causes cosmetic problems. As a result, many patients want a less invasive procedure. Recently, the use of US-VAE for removal of benign breast masses has been on the increase because the procedure is simple, less time-consuming, low cosmetic problem and feasible in the outpatient setting with the use of local anesthesia [1,2,8]. However, some lesions after performing US-VAE require further re-excision according to final pathology. Although US-VAE is very simple procedure, re-excision after US-VAE would be burden in patients and physician. Therefore, accurate diagnosis before US-VAE assessment of breast lesions is important to avoid open surgical excision. Diagnosis of benign breast disease involves a combination of clinical examination, imaging, and needle biopsy. Clinical diagnoses alone are often unreliable and will not exclude malignancy in either the younger or older patient, while tissue biopsy is the most accurate means of establishing the diagnosis. Currently, the gold standard for breast biopsy procedures is needle biopsy. The goal of these minimally invasive biopsy procedures is to reduce the invasiveness and also to reduce the procedural costs without sacrificing accuracy. In particular, image-guided FNAB or CNB has become an established technique. US-FNAB and CNB are less expensive than surgical biopsy in patients with and without cancer. It has been reported that US-CNB yielded a 56% decrease in the cost of diagnosis in comparison with surgical biopsy [9].
The diagnostic accuracy of CNB has been actively evaluated and several reports have published. The studies were reported from multiple institutions over the last decade, and have shown good concordance of histologic diagnosis between CNB and surgical biopsy. Usami et al. [10] reported high concordance between the diagnoses from CNB and surgical biopsy ranging from 91% to 100%. Nguyen et al. [11] suggested that the sensitivity and specificity of CNB were 99%, 100%, respectively. Fajardo et al. [12] also reported the accuracy of CNB and sensitivity, specificity, and accuracy of CNB were 91%, 100%, and 98%, respectively. Another long term, multi-institutional prospective study estimated that the sensitivity, specificity, and accuracy of CNB were 91-92%, 98-100%, and 96-97%, respectively [13]. The Core Biopsy after Radiological Localization (COBRA) study also showed high diagnostic accuracy. The sensitivity and specificity for CNB in this trial were 97% and 99% [14]. More recent study suggested that sensitivity and specificity of CNB were 98% and 99%, respectively [15].
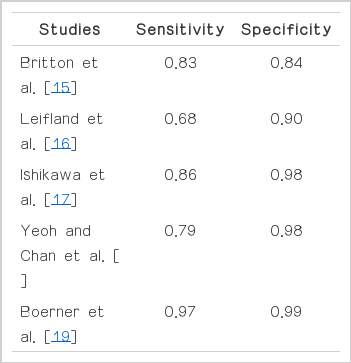
FNAB can also be diagnostic and may be most popular diagnostic tool. FNAB is rapid, simple, and less invasive. The confirmation of cystic lesions is excellent. Britton [16] reviewed 17,108 biopsies from 31 papers. The study concluded that the average sensitivity of US-guided FNAB was 83.1% and average specificity was 84%. Leifland et al. [17] reported that sensitivity and specificity of FNAB were 68% and 90%, respectively. Other study suggested that the sensitivity, specificity, and accuracy of FNAB were 86.3%, 98.2%, and 93.2%, respectively [18]. Yeoh and Chan [19] reviewed 1,533 FNA cases and reported that overall sensitivity was 79%, specificity 98%. Another author suggested sensitivity and specificity of FNAB were 97.1% and 99.1% [20]. In our study, the sensitivities of FNAB in benign lesion were 99% (Table 4).
As above described, these techniques have showed the good accur-acy in many studies; however, they have false-negative and underestimation of diagnosis of breast lesion. Therefore, there is the possibility of delay in the diagnosis of breast cancer. However, the false-negative rates were relative low in many trials. US-guided biopsy false-negative rates has been reported range 0.6% to 22.2% [21]. Of these techniques, in case of US-CNB, overall false-negative rate has been established a range from 0% to 9% [9]. Studies of Helbich et al. [22], Smith et al. [3], and suggested the false-negative of CNB was 0%. Nguyen et al. [11] reported that high sensitivity and specificity, however, false negative rate of CNB was 1.7%. The lowest false-negative of US-guided CNB among triple assessment (US-guided, stereotactic and clinical) was reported in 1.7% in other studies. And other studies about false-negative rate of CNB were described detail in Table 4 [23].
Morris et al. [24] suggested that 29% of breast lesions are heterogeneous yielding different histologic results from targeted center and periphery, thus CNB sampling only part of a heterogeneous mass result in a misdiagnosis. Joshi et al. [25] provided that CNB findings of ADH underestimate the diagnosis of malignancy by 18% to 88%. Other study showed high sensitivity (94-95%), good specificity (94%), and very low inadequacy of CNB; however, 4.4% of false-negative rate was showed and also CNB was associated with a significant rate of underestimation of malignancy (26.6%) when sampling calcifications, and has relatively low accuracy when sampling mammographic distortions [21].
FNAB also has the false-negative rate in the presence of cancer is 6% to 11% [15]. Factors that may influence these results include the experience of the clinician and pathologist, and the size and histological type of the tumor. Inadequate sampling is a contributory factor to the reduced sensitivity of tissue [15]. Boerner et al. [20] reported that false-negative of FNAB was 3.7%. Another study showed false-negative of FNAB in 2.2% [18].
Many studies about accuracy of US-CNB and FNAB have showed high sensitivity, specificity, and relative low false-negative rate. And the studies have established CNB and FNAB are good alternative technique of open biopsy in diagnosis of breast lesion. However, although the false-negative rate is relative low, careful adherence to the principles of multiplicinary assessment support (clinical, radiological, pathological) is essential to avoid delay in diagnosis of breast cancer in patients with false-negative biopsies. And to avoid underestimation, rebiopsy and close work-up should be considered according to radiologic and clinical findings. The study about effectiveness of concurrent CNB and FNAB suggested that the false-negative rate could be reduced by 44% compared with the rate obtained by CNB only (2.5-1.1%) [15]. Therefore, concurrent procedure of CNB and FNAB can be considered as a better method in diagnosis of breast suspicious lesion to reduce false-negative and underestimation rate.
The debate of further excision necessity after US-VAE in premalignant lesions or lesions which are difficult to differentiate with malignancy has not been established yet. In this study, premalignant lesions were not considered further excision if the lesion was completely excised by US-VAE. One of premalignant breast disease, ADH is associated with a fourfold to fivefold increased risk of breast carcinoma [26]. The authors suggested that variables predicting for malignancy is associated with previous contralateral breast cancer, family history of breast cancer, markedly atypical hyperplasia. Therefore, the study proposed that mild ADH found on US-VAE, not associated with a personal or family history of breast cancer, may not need re-excision if all calcifications have been removed. Adrales et al. [26] and Grady et al. [27] also support above suggestion.
In our study, accuracy of FNAB in benign lesion is 99%, even though the procedures were processed by two surgeons. The reasons are that there are two skillful surgeons who had experience more than 5,000 cases. And also we used Fan technique which means that we obtain enough tissues or cells from several directions of the masses when performing FNAB. Therefore, before performing US-VAE, detail and exact FNAB can help to obtain high accuracy diagnosis of breast lesions. However, we acknowledge that this study is limited by a lack of long-term follow-up. And so, it is difficult to confirm complete removal of pathological abnormalities, especially premalignant lesions. Therefore, the risk of a histologic upgrade could be underestimated.
US-VAE is a fairly recent minimally invasive excision technique for removing benign breast lesion. However, further surgical excision according to final pathologic results after performing US-VAE is sometimes necessary. It is difficult to know when primary surgical excision is better than US-VAE. Therefore, the accurate diagnosis of breast lesion to decide for taking US-VAE and to avoid open surgical excision is important. In our study, further excision rate was very low (0.9%). FNAB is also good minimally invasive procedure instead of invasive core needle biopsy for reducing further excision rate after US-VAE.
Notes
CONFLICT OF INTEREST
The authors declare that they have no competing interests.