Clinicopathologic Features of Pleomorphic Invasive Lobular Carcinoma: Comparison with Classic Invasive Lobular Carcinoma
Article information
Abstract
Purpose:
The purpose of this study was to identify the clinical and pathological factors that differentiate pleomorphic invasive lobular carcinoma (PILC) from classic invasive lobular carcinoma (CILC).
Methods:
We retrospectively reviewed the medical records of 65 patients (4.0% of all invasive breast cancer patients) who underwent surgical excision for invasive lobular carcinoma (ILC) between January 2000 and November 2013. All 65 patients were diagnosed with ILC with negative immunohistochemical staining for E-cadherin in the tumor cells. All hematoxylin and eosin slides of the previously diagnosed ILC were reviewed and confirmed by two expert pathologists and we compared the clinicopathologic features between CILC and PILC.
Results:
CILC was found in 46 cases and PILC, in 19 cases. Of the mammographic findings, a mass or asymmetric density was the most common feature (42.3% of all ILC patients). The most common ultrasonographic feature was a mass (94.9% of all ILC patients). Tumor multiplicity was noted in 10 patients (15.4%) among all ILC patients; eight patients (17.4%) had CILC and two patients (10.5%) had PILC. PILC patients had more grade III tumors (66.7% vs. 8.7%, p=0.002) and a higher Ki-67 labeling index (55.6% vs. 18.6%, p=0.004) than those with CILC. There were no statistical differences in the type of combined in situ component, extensive intraductal component, tumor size, lymphovascular invasion, stage, hormone receptor status, human epidermal growth factor receptor 2 status, distribution of intrinsic subtype, or imaging findings. Moreover, there was no significant difference in survival between CILC and PILC.
Conclusion:
PILC showed more pathological aggressiveness than CILC in terms of tumor grade and Ki-67 index.
INTRODUCTION
Invasive lobular carcinoma (ILC) is the second most common subtype of breast cancer, accounting for 5% to 15% of all invasive breast cancers [1,2]. It was first described by Foote and Stewart in the 1940s, and it is characterized by loss of cell-to-cell adhesion molecules and E-cadherin protein expression [3,4]. ILC is subdivided into classic ILC (CILC) and non-classic ILC, which is known as pleomorphic ILC (PILC). PILC was first described in 1987 by Page as a distinct morphologic variant of CILC [5,6]. Unlike CILC, which is characterized by small, relatively uniform tumor cells with round nuclei and a single file growth pattern, PILC usually has enlarged and irregular nuclei, increased mitotic activity, and abundant eosinophilic cytoplasm (Figure 1) [6-8]. Studies have shown that the features of PILC include lower estrogen receptor (ER) expression, higher histologic grade, overexpression of human epidermal growth factor receptor 2 (HER2), higher Ki-67 labeling index, and poorer prognosis compared to CILC, but most of these studies have limitations owing to their small sample size [6,9,10]. The purpose of the current study was to identify the differences in the clinical and pathological factors that distinguish PILC from CILC.
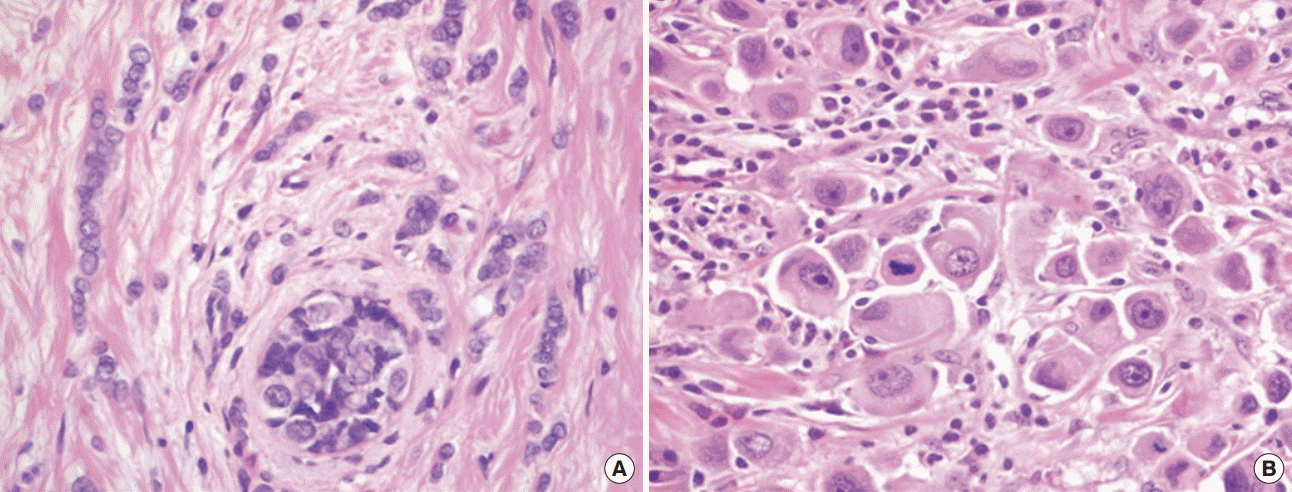
Microscopic findings of invasive lobular carcinoma. (A) Characteristically, classic invasive lobular carcinoma has a single file growth pattern. The tumor cells are small and uniform, with smooth nuclear membranes and indistinct nucleoli. (B) Pleomorphic invasive lobular carcinoma showing discohesive tumor cells. Tumor cells have markedly enlarged hyperchromatic nuclei and abundant eosinophilic cytoplasm. Additionally, prominent nucleoli and atypical mitotic figures are frequently observed (H&E stain, ×400).
METHODS
Patients
This study was approved by the Institutional Review Board of Dongsan Medical Center (DSMC201603047). We retrospectively reviewed the medical records of 65 patients (4.0% of all invasive breast cancer patients) who underwent surgical excision for ILC between January 2000 and November 2013. All 65 patients were diagnosed with ILC with negative immunohistochemical staining for E-cadherin in the tumor cells. All hematoxylin and eosin slides of previously diagnosed ILC were reviewed and confirmed by two expert pathologists. The diagnostic criteria of PILC are defined as histological findings such as the classic discohesive growth pattern noted in CILC, marked enlarged nuclei (3 to 4 times the size of lymphocytes) with irregular nuclear membranes, prominent nucleoli, increased mitotic counts, and moderate to abundant eosinophilic or amphophilic cytoplasm. The clinicopathological features that were recorded included patient age, surgical method, tumor stage, imaging findings, tumor multiplicity, extensive intraductal component, combined in situ lesions, histologic grade, lymphovascular invasion, hormone receptor status, HER2 status, Ki-67 labeling index, and intrinsic subtype.
Immunohistochemistry
All immunohistochemical staining for ER, progesterone receptor (PR), HER2, and Ki-67 was performed using two different methods. The previous method used from 2000 to 2008 was manual detection of antibodies. Since then we have used the automated BenchMark platform (Ventana Medical Systems Inc., Tucson, USA), according to the manufacturer’s recommendations. Positivity of ER and PR was defined when nuclear staining was present in more than 1% of tumor cells, according to the American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) guidelines. The status of immunohistochemical staining of HER2 was scored in four categories according to the ASCO and CAP guidelines [11]. Scores 0 and 1+ were regarded as negative and score 2+ was determined to be equivocal. Score 3+ was considered as positive [12]. The Ki-67 labeling index was considered positive if nuclear immunostaining was detected in 20% or more of the tumor cells [13,14].
Statistical analysis
Recurrence was confirmed by direct biopsy or radiologic findings. The chi-square test and Fisher exact test were used to compare clinicopathological variables between each group. The Kaplan-Meier method was used for estimation of disease-free survival (DFS) and overall survival (OS), and the log-rank test was used to verify significant differences between survival outcomes. DFS was calculated from the operation date to the first locoregional or distant recurrence. Statistical analyses were performed with SPSS software, version 20.0 (IBM Corp., Armonk, USA). A value of p<0.05 was considered to be statistically significant.
RESULTS
CILC and PILC were identified in 46 and 19 cases, respectively. Synchronous bilateral breast cancer was identified in four patients among all 65 ILC cases; two patients had bilateral ILC (one was bilateral CILC, and the other was bilateral PILC), and two had ILC in one breast and invasive ductal carcinoma (IDC) in the other breast.
The most common ultrasonographic feature was a mass (95.2% and 94.1% in CILC and PILC, respectively). A mass or asymmetric density was the most common mammographic feature (42.1% vs. 42.9%). Negative findings were observed in 10 patients (23.7% vs. 7.1%), and microcalcification only was observed in six (7.9% vs. 21.4%). No cases with negative ultrasonographic findings or mammographic microcalcification were noted. Breast-conserving surgery was performed in 21 patients among all ILC patients, 17 (37.0%) of whom had CILC and four (21.1%) who had PILC. Mastectomy was performed in a total of 44 patients, including 29 patients (63.0%) with CILC and 15 (78.9%) with PILC. Stage I disease was noted in 27 patients (CILC vs. PILC, 45.7% vs. 31.6%), stage II in 22 patients (37.0% vs. 26.3%), and stage III in 16 patients (17.4% vs. 42.1%). There were no statistically significant differences in patient age, surgical method, tumor stage, or imaging findings between the CILC and PILC groups (Table 1).
Tumor multiplicity was noted in 10 patients (15.4%) among all ILC patients; eight patients (17.4%) had CILC and two patients (10.5%) had PILC. Four patients (CILC vs. PILC, 6.5% vs. 5.3%) had an extensive intraductal component, and 40 (63.1% vs. 57.9%) had an in situ component. Lymphovascular invasion was noted in 27 patients (23.8% and 41.2% in CILC and PILC, respectively). There were no statistically significant differences in any of these features.
Hormone receptor expression was positive in 55 patients (90.2%), and slightly more patients were hormone receptor-positive in the CILC group, but this difference was not statistically significant (CILC vs. PILC, 93.0% vs. 83.3%, p=0.246). HER2 expression was positive in nine patients (14.8%) among all ILC patients; seven patients (16.3%) had CILC and two patients (11.1%) had PILC, which was not significantly different. Among all patients, four patients (6.6%) had the triple-negative breast cancer (TNBC) type, two with CILC and two with PILC. More non-TNBC type cases were noted in the CILC than in the PILC group, but the difference was not statistically significant (p=0.353).
There were statistically significant differences in two pathologic features, the histologic grade and the Ki-67 index. The PILC group showed more grade III tumors (66.7% vs. 8.7%, p=0.002) and a higher Ki-67 labeling index (55.6% vs. 18.6%, p=0.004) than the CILC group (Table 2).
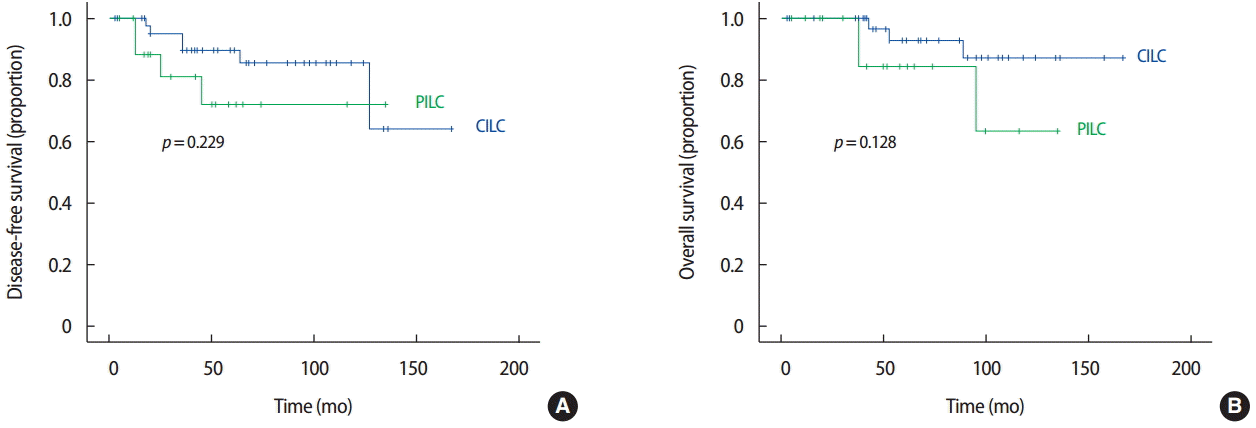
The median follow-up period for all patients was 53 months (range, 3–167 months; CILC vs. PILC, 60 months vs. 50 months), and the follow-up period for 10 patients who experienced recurrence was 71 months (range, 16–158 months; 71 months vs. 66 months). The 5-year DFS rate was 84.6% (89.6% vs. 71.9%, p=0.229), and the 5-year OS rate was 90.9% (93.1% vs. 84.6%, p=0.128). There were no statistically significant differences in DFS or OS between the CILC and PILC groups (Figure 2). However, PILC showed a worse tendency in DFS and OS compared with CILC.
DISCUSSION
ILC was first described as a variant form of IDC in 1941. ILC arises from the acinar epithelium of the mammary gland and occasionally appears similar to the normal parenchyma of dense breasts [15]. Even though ILC accounts for less than 15% of all breast cancers, numerous studies on ILC have described its different clinical features and treatment results in comparison with those of IDC [2,16,17]. Based on these studies, patients with early-stage ILC can be treated with breast-conserving surgery and postoperative radiotherapy instead of mastectomy, and patients with advanced-stage ILC are treated with aggressive surgery and adjuvant systemic therapy. Owing to these developments in disease management, the prognosis of ILC has improved over time [18-21]. However, patients with ILC still have a poorer prognosis compared to those with IDC; ILC results in poorer DFS and OS compared to IDC [22]. The re-excision rate after breast-conserving surgery for ILC is twice that after surgery for IDC [23]. As such, more accurate diagnosis and management of ILC are needed.
Although it is difficult to classify and subtype ILC, studies have demonstrated the histological heterogeneity of ILC, which has two subtypes, CILC and PILC [2,9]. Histologically, CILC is characterized by typical small-to medium-sized uniform tumor cells arranged linearly. The tumor cells are frequently arranged around ducts and lobules [9,24]. By contrast, PILC has atypical tumor cells with marked cellular pleomorphism and large, eccentric, hyperchromatic nuclei [5,22]. PILC is a rare disease, accounting for less than 1% of all invasive breast cancers [10]. In our study, PILC represented 1.1% of all invasive breast cancer cases.
The mammographic appearance of ILC is often a spiculated mass lesion not associated with microcalcification. The measurement of tumor size is incorrect when it is determined using mammography, and the mammographic features are not useful for distinguishing between the ILC subtypes [25]. Additionally, the ultrasonographic findings of ILC do not provide information regarding tumor size or subtypes [26]. In a few studies, magnetic resonance imaging has proven useful for accurately planning surgical treatment, but it is not useful for distinguishing between the two subtypes of ILC [27,28].
Ciobanu et al. [9] studied 25 cases of ILC (11 cases of CILC and 14 cases of PILC) in 2012. Compared with CILC, PILC was predominantly diagnosed in older individuals (median age of 59 years), with an advanced stage (stage III and IV) and lymph node involvement. There was no significant difference in the hormonal and HER2 profiles between the two subtypes. Orvieto et al. [24] studied 530 patients with ILC (301 patients with CILC and 229 with PILC) in 2008, and significant prognostic factors were verified, including tumor size, lymph node metastasis, and hormonal status. The patients with PILC had more advanced-stage tumors, lymph node metastasis, and distant metastasis, and they demonstrated reduced DFS and OS compared to those with CILC. However, our study demonstrated no statistically significant differences in DFS or OS between the groups.
Rakha et al. [6] reported the findings of 202 cases of grade 2 or 3 PILC categorized according to their histologic components. Based on their results, the PILC subtype has no prognostic usefulness to histologic grade in ILC. Jacobs et al. [10] reported 58 cases of CILC and seven cases of PILC that were analyzed according to their clinicopathological features and biomarker expression. PILC had a more aggressive histologic grade, higher proliferative index of Ki-67, and negative expression of ER.
We should consider the limitations of the current study. The data of this study were reviewed retrospectively. Even though the follow-up period was relatively long, the number of patients within a single center was small due to the rarity of disease. Thus, further prospective multicenter studies are needed for identification of prognostic factors in ILC patients.
In conclusion, PILC showed more pathological aggressiveness than CILC in terms of tumor grade and Ki-67 index, but other prognostic factors such as the hormone receptor status and HER2 status were not different between the two subtypes of ILC.
Notes
The authors declare that they have no competing interests.