Very Rare Case of Spindle Cell Carcinoma of Breast in Male
Article information
Abstract
Metaplastic breast carcinoma is uncommon, constitutes ≤5% of all breast cancers, and is classified into various subtypes with varying incidences. Of these subtypes, spindle cell carcinoma represents approximately 0.3% of all invasive breast carcinomas in women. The spindle cell carcinoma subtype of metaplastic breast carcinoma is typically triple negative and has distinct clinical, radiological, and pathological characteristics. To date, there is no effective treatment for this malignancy. Herein, we report a case of spindle cell carcinoma of the breast in a 71 year-old man who presented with a palpable mass in his left breast. Breast ultrasonography revealed a 1.1×2.6 cm hypoechoic well-demarcated ovoid mass. The patient underwent excisional biopsy. Pathological findings indicated a diagnosis of spindle cell carcinoma of the breast, and the patient underwent a modified radical mastectomy. The final pathological report indicated a 6.5×3.0 cm malignant spindle cell tumor.
INTRODUCTION
Spindle cell carcinoma of the breast is a rare subtype of metaplastic carcinoma, and is characterized by a predominance of spindle cells [1,2]. These cancers represent approximately 0.3% of all invasive breast carcinomas, and the prognostic significance of this subtype is uncertain [1]. However, several studies have suggested that spindle cell carcinomas display a more aggressive behavior than other types of breast cancers, primarily owing to the lack of specific treatment options and the high recurrence risk [1-4]. Herein, we present a case of spindle cell carcinoma of the breast in a man, and describe a review of the literature on this rare but interesting disease.
CASE REPORT
Herein, we report a case of spindle cell carcinoma of the breast in a 71-year-old man, who presented with a palpable mass in his left breast. He underwent breast ultrasonography (US), which confirmed the presence of the mass. The patient had undergone prostatectomy for prostate cancer 2 years previously. No malignant calcification, skin thickening, or nipple retraction was observed on the mammogram. Diagnostic breast US revealed a hypoechoic solid ovoid mass measuring approximately 1.1 × 2.6 cm at the 11 o’clock position in the left breast (Figure 1). The US findings suggested a lipoma or fibroadenoma. Therefore, open excisional biopsy was performed, which revealed a malignant spindle cell tumor of the breast with a positive resection margin. After open excisional biopsy, the tumor size measured by the pathologist was 2.0 × 2.0 cm. The excised specimen did not contain the whole tumor. Two weeks later, modified radical mastectomy and partial resection of the pectoralis major muscle (owing to the invasion of malignant cells observed on the intraoperative frozen biopsy) were performed. The biopsy revealed a malignant spindle cell carcinoma of the breast, which was triple negative (negative for estrogen receptor [ER], progesterone receptor [PR], and human epidermal growth factor receptor 2 [HER2]). No regional lymph node metastasis (0 of 17 axillary lymph nodes) was detected. The true tumor size measured by the pathologist was 6.5 × 3.0 cm, and the excised specimen consisted of the whole tumor. The patient’s biopsy specimen revealed a tumor composed of breast tissue exhibiting features of spindle cell carcinoma. The histological analysis of this patient revealed ill-defined lesions and occasional bands of hyaline collagen separating these spindle cells (Figure 2A), consisting mostly of satellite spindle cells with plump oval nuclei showing frequent mitoses and scattered chronic inflammatory cells (Figure 2B), isolated islands of bland squamous epithelium, and trapped benign ductal structures were also identified. Immunohistochemical analysis revealed the neoplastic spindle cells to be positive for vimentin expression (Figure 2C).
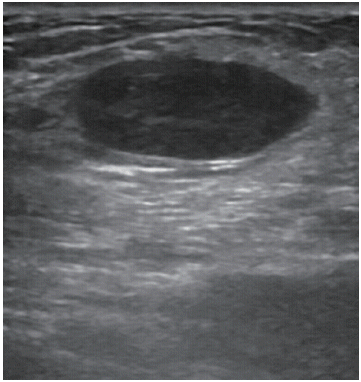
A diagnostic breast sonogram showing a hypoechoic solid, ovoid mass measuring approximately 2.6×1.1 cm in diameter at the 11 o’clock position of the left breast.

Microscopic findings. (A) Spindle cells with pleomorphism and high nuclear-cytoplasmic ratio are arranged in storiform pattern (H&E stain, ×200). (B) Spindle shaped malignant cells are arranged in storiform pattern. Frequent mitosis are noticed (white arrows) (H&E stain, ×400). (C) Malignant cells are stained brown (red arrow), consistent with positive vimentin expression (immunohistochemical stain, ×200).
Four weeks after the mastectomy, a chemotherapy regimen of 5-fluorouracil plus doxorubicin and cyclophosphamide (FAC) was initiated. Three cycles of chemotherapy were administered and positron emission tomography/computed tomography (PET/CT) was performed during follow-up, which did not show any metabolic evidence of recurrence or metastasis in the patient’s chest or other regions of his body. An additional three cycles of chemotherapy were administered following the initial PET/CT, and there was no significant change on the second PET/CT scan obtained 6 months later. The patient recovered well; approximately 10 months since his diagnosis, there is no evidence of cancer recurrence and the patient continues to receive follow-up examinations in the breast clinic.
DISCUSSION
The clinical presentation of spindle cell carcinoma of the breast is similar to that of more commonly observed types of mammary carcinoma. Similar to other breast cancer types, the mean age at diagnosis of spindle cell carcinoma of the breast is 62 years in women and 67 years in men [5]. Spindle cell carcinoma often presents as a rapidly growing palpable mass with high density on a mammogram [1]. Our patient was diagnosed based on the diagnostic breast US findings and the presence of a palpable mass. The typical clinical pattern of these tumors, however, is not clear. Clinicopathologically, spindle cell carcinoma is characterized by a larger tumor size and significantly less lymph node involvement compared to the more common primary breast cancers [4]. One study showed a mean tumor size of 4.6 cm (range, 1.5–31 cm), which is larger than the size of the more common breast cancers on presentation [1]. Furthermore, hematogenous spread of these tumors to the lungs and bones is more commonly observed than lymphatic spread [1-4]. Axillary node metastasis reportedly occurs in approximately 0% to 26% of patients, which is lower than the rate reported for typical mammary carcinomas [1]. The present patient had no axillary node metastasis. For most patients with spindle cell carcinoma, sentinel node biopsy seems to be a reasonable diagnostic tool that can be used to avoid premature and unnecessary axillary surgery during treatment [1]. However, men with breast cancer are more likely to have lymph node involvement and a higher stage at diagnosis, and spindle cell carcinomas show more aggressive behavior than other subtypes [1-5]. Therefore, we opted to perform axillary lymph node dissection.
Accurate pathological diagnosis is vital because the differential diagnosis of spindle cell carcinoma of the breast may include benign and malignant entities such as fibromatosis, phyllodes tumor, inflammatory myofibroblastic tumor, and primary low-grade sarcoma. Histopathological and immunohistochemical profiles play an important role in differentiating between the different tumor types [1]. In our particular case, the results of the patient’s histological analysis were consistent with the histopathological changes previously described in cases of low grade fibromatosis-like spindle cell carcinoma of the breast [6,7].
Immunohistochemical staining demonstrates neoplastic spindle cells to be diffusely positive for vimentin, pan-keratin, and high molecular weight cytokeratin, but focally weak for smooth muscle actin. Immunohistochemical staining for other markers such as desmin, cytokeratin 7, and epithelial membrane antigin is negative within neoplastic spindle cells, whereas staining for caldesmon highlights the presence of myoepithelial cells surrounding entrapped ductal structures [1]. In our particular case, immunohistochemical analysis revealed neoplastic spindle cells to be negative for ER, PR, and HER2, which are typical findings of triple negative spindle cell carcinoma [1]. The 2003 World Health Organization pathological classification of tumors of the breast [8] includes the following under the category of metaplastic carcinoma: (1) pure epithelial metaplastic carcinoma, which includes squamous cell carcinoma, adenocarcinoma with spindle cell metaplasia, adenosquamous carcinoma, and mucoepidermoid carcinoma; and (2) mixed epithelial/mesenchymal metaplastic carcinoma. Therefore, the present case can be classified as metaplastic adenocarcinoma with spindle cell metaplasia.
On mammogram, spindle cell carcinomas predominantly manifest as circumscribed round, oval, or lobular, noncalcified high-density masses. In contrast, invasive ductal carcinomas typically present with an irregular shape and speculated margins [9]. Carcinomas with a mixture of growth patterns of metaplastic and unspecified invasive carcinomas may show concurrence of a circumscribed portion with a speculated portion, an important feature that can help in distinguishing metaplastic carcinoma from other circumscribed breast masses [9]. On breast US, spindle cell carcinomas present as round or lobular masses with well circumscribed or microlobulated margins and show complex internal echogenicity, with solid and cystic components that are related to necrosis and cystic degeneration [10]. These tumors frequently demonstrate posterior acoustic enhancement, unlike the posterior acoustic shadow frequently associated with invasive ductal carcinomas. These combined mammographic and ultrasonographic features can lead to misinterpretation of these tumors as benign or probably benign (Breast Imaging-Reporting and Data System category 2 or category 3 lesions), which could potentially delay diagnosis [11]. In this particular case, breast US revealed solid, oval-shaped, well-circumscribed masses without posterior acoustic enhancement. Awareness of this commonality in imaging findings can be used to differentiate between metaplastic carcinomas and probably benign lesions, leading to timely biopsy and ancillary testing of histologic specimens.
Spindle cell carcinoma is typically triple negative, meaning that these cancers are often resistant to established breast cancer-targeted therapies such as antiestrogen therapies and trastuzumab. Spindle cell carcinomas of the breast are generally reported to be less responsive to conventional regimens used for treating typical adenocarcinomas of the breast [1]. Therefore, patients with spindle cell carcinoma should be considered candidates for innovative therapeutic regimens. Furthermore, clinicians should be aware that patients with this type of tumor have very limited treatment options and require close monitoring. When compared to typical breast carcinoma, spindle cell carcinoma is associated with a significantly decreased overall survival rate and shows highly aggressive behavior, possibly owing to the lack of specific treatment options and high recurrence risk [1]. In a study of 30 patients with spindle cell carcinoma of the breast conducted at MD Anderson, two patients developed lung metastases within 3 years of initial diagnosis [12]. Spindle cell carcinoma carries a 5-year survival rate of 64% and >50% of spindle cell carcinomas are associated with either local or distant metastases (or both) within 5 years, suggesting poor prognosis [1].
Spindle cell carcinoma is a relatively rare but aggressive form of breast cancer. Most previous studies of this malignancy had a small sample size and inconsistent results. The definitive prognosis of spindle cell carcinoma of the breast remains disputed, but considering its typical presentation as a triple negative tumor and its potential for distant metastasis, close monitoring is required. Familiarity with the distinct clinical, pathological, and radiological presentation of these tumors is needed for accurate diagnosis. Undoubtedly, further research is warranted to determine the characteristics of this subtype of breast carcinoma and to identify more specific treatment options. Because the result of the biopsy for our case was a triple-negative carcinoma, FAC regimen was used for adjuvant chemotherapy after surgery. However, the role of adjuvant chemotherapy and radiation for this tumor has also been unclear [13,14]. More number of clinical studies is needed in order to diagnose and treat this disease optimally.
Notes
The authors declare that they have no competing interests.