Clinicopatholgic Characteristics to the Relapse on Ductal Carcinoma In Situ: Ki-67 Index as the Most Influential Prognostic Factor
Article information
Abstract
Purpose
The biggest concern related to ductal carcinoma in situ (DCIS) is local recurrence and recurrence patterns. The purpose of this study was to investigate the relationship between clinicopathological factors and relapse in patients treated with DCIS.
Methods
We reviewed medical records of 104 patients who were diagnosed as DCIS between January 1999 and December 2015 at a single institute. We compared and analyzed clinicopathological factors such as age at diagnosis, preoperative lesions on ultrasonography, preoperative tumor markers, operation methods in the breast, histological grade, nuclear grade, resection margin, comedonecrosis, estrogen receptor/ progesterone receptor expression, human epidermal factor receptor 2/neu expression, Ki-67, postoperative implementation of adjuvant hormonal therapy, and radiotherapy by dividing them into recurrent and non-recurrent groups.
Results
Seventeen patients (16.3%) of 104 patients relapsed in the ipsilateral or contralateral breast. The median follow-up period of non-relapsed group was 4.9 years (range, 0.5–19.15) and the median follow-up period of relapsed group was 3.5 years (range, 1.4–14.13). Clinicopathological factors that were significantly related to relapse were nuclear grade (p=0.022) and Ki-67 (p=0.025) based on the results of chi-square or Fisher’s exact analysis. In multivariate analysis using logistic regression, Ki-67 (p=0.021) was significantly associated with DCIS relapse.
Conclusion
This study suggested that the higher Ki-67 over 14% was strongly associated with DCIS relapse.
INTRODUCTION
Breast cancer is the most common malignancy among women in Korea. Since 2016, it has maintained its top position with a cancer incidence of nearly 20% [1]. Korean National Health Insurance Service (NHIS) system recommends all Korean women to receive screening mammography every two years from their 40s if they are registered to get the NHIS. These screening mammography contributed to early detection of breast cancer and also affected the incidence of breast cancer [2,3]. It is well known that mortality rate from breast cancer reduced in asymptomatic women aged 40 to 69 years who underwent screening mammography every two years, whereas mortality from breast cancer did not decrease in women over 70 years of age [4].
According to the data from the Korea National Cancer Incidence Database and annual report of Statistics committee in Korean Breast Cancer Society (KBCS), the incidence of ductal carcinoma in situ (DCIS) has been gradually increasing 10.1 times from 1.6 in 2000 to 16.1 in 2017 per 100,000 population [5]. In particular, many portions of DCIS may appear only as clustered microcalcifications without mass density on mammography [6]. As screening mammography has become widespread, more DCIS lesions are being detected. DCIS is a non-invasive, non-obligate precursor of invasive breast cancer [7]. Patients with DCIS have a good prognosis; however, invasive recurrence is associated with mortality [8]. Therefore, it is important to identify patients at risk for DCIS recurrence. This study was conducted to investigate clinical pathological factors associated with recurrence in DCIS patients.
METHODS
We reviewed medical records of 140 patient who were diagnosed as DCIS in initial pathology report after needle biopsy and excluded 32 patients who reported as invasive ductal carcinoma (IDC) in the final pathology report after surgery. Consequently, a number of 104 patients with DCIS were included in this study. Patients with DCIS were diagnosed and treated at a single medical institute between January 1999 and December 2015. The patients were classified into two groups according to the presence or absence of recurrence (non-relapsed group vs. relapsed group). We analyzed demographic characteristics and clinic-pathological factors in both non-relapsed group and relapsed group. This study was approved by the Institutional Review Board of Inje University Saynggye Paik Hospital (IRB No. SGPAIK 2020-07-008-001) and was performed in compliance with the ethical standards of the Declaration of Helsinki. The requirement for informed consent was waived owing to the retrospective nature of the study.
Clinical variables were composed of age at diagnosis, preoperative lesions on ultrasonography, tumor markers such as Cancer Antigen 15-3 (CA15-3) and carcinoembryonic antigen (CEA), which were performed preoperatively, and operation methods in the breast. Age at diagnosis was divided into two groups based on age 50 because the average age of menopause in Korean women is 50 years. Preoperative lesions were divided into three groups: 1) mass on sonography without calcification on mammography, 2) mass on sonography with calcification on mammography, 3) no mass on sonography and only calcification on mammography. Tumor markers such as CA15-3 and CEA were divided into normal and elevated groups based on normal levels in preoperative blood tests like < 30 U/mL and 6 ng/mL, respectively. All patients underwent breast-conserving surgery (BCS) or mastectomy.
Pathologic variables were including nuclear grade (NG), histological grade (HG), comedonecrosis, resection margin, estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2/neu) overexpression, and Ki-67 index (%). DCIS is divided into three histological tumor grades according to NG and comedonecrosis. Tumors with non-high NG and without necrosis were classified as low-grade, those with non-high NG with necrosis were classified as intermediate grade, and those with high NG with or without necrosis were classified as high-grade disease. According to results of the pathology reports, any cancer cell existing on the margin was defined as an involved margin. A closed margin was defined when cancer cells were within 1 mm of the resection margin. In other cases, the resection margin was classified as clear. In this study, a positive resection margin was defined as involved or closed groups, and a negative one was defined as clear group [9].
Immunohistochemistry (IHC) analysis for ER, PR, HER2/neu, and Ki-67
IHC analysis was also performed on the histopathology of cancer cells. The latest American Society of Clinical Oncology guidelines for the detection and scoring of ER, PR, and HER2/neu statuses were followed [10]. ER and PR were scored as 0, 1+, 2+, and 3+ according to staining intensity with a description of the percentage related to the proportion of stained nuclei in 10 high power fields. ER and PR positivity was defined as any positive score or a percentage greater than zero. We converted the intensity scores and proportion percentages into the Allred score. The IHC staining for HER2/neu was graded as follows: 0, 1+, 2+, and 3+. Intensity scores of 0 or 1+ were designated as negative expression, 2+ was designated as equivocal, and 3+ was designated as positive expression for HER2/neu. IHC staining for Ki-67 divided into low (≤ 14%) and high (>14%) group, the standard of the existing PAM50 intrinsic multigene molecular test classification [11,12].
BCS and mastectomy are widely acknowledged for the management of DCIS. Patients diagnosed with DCIS undergo BCS or mastectomy according to the extent of DCIS. Although axillary lymph node staging has not been routinely performed in the majority of DCIS patients, we considered sentinel lymph node biopsy if mastectomy is planned for extensive malignant calcifications or if BCS is planned for relatively large DCIS, or high-grade DCIS, or suspicious microinvasion.
Postoperatively, radiotherapy was considered for the ipsilateral breast in patients who underwent BCS surgery. Adjuvant hormonal therapy was also performed only when the patients were diagnosed as ER- or/ and PR-positive. Adjuvant hormonal therapy was routinely prescribed with tamoxifen irrespective of whether the patients received radiotherapy. All patients underwent mammography, physical examination, chest radiography, and breast ultrasonography during the follow-up period. The total number of invasive and noninvasive recurrence events were monitored.
Statistical methods
Pearson chi-square or Fisher’s exact test was used for categorical variables. Logistic regression analysis was performed on the valid variables in the univariate analysis. All statistical analyses were performed using IBM SPSS Statistics version 23.0 software (IBM Corp., Armonk, USA). Statistical significance was set at p< 0.05.
RESULTS
Clinical and pathological characteristics of non-relapsed and relapsed DCIS patients are shown in Table 1. In the univariate analysis, among the 104 patients who underwent surgery, 17 patients (16.3%) recurred as DCIS or invasive carcinoma in the ipsilateral or contralateral breast. The median follow-up period of non-relapsed group was 4.9 years (range, 0.5-19.15) and the median follow-up period of relapsed group was 3.5 years (range, 1.4-14.13). There were no statistically significant differences between non-relapse and relapsed group in age at diagnosis (p= 0.430), preoperative CA15-3 (p=1.000) and CEA (p= 0.156), and operation method (p= 0.456). Besides, there were no statistically significant differences between non-relapse and relapsed group in HG (p = 0.095), resection margin (p = 0.257), and comedonecrosis (p=1.000). However, there was statistically significant difference between non-relapse and relapsed group in NG (p= 0.022).
In addition, there were no statistically significant differences between non-relapse and relapsed group in ER (p= 0.475), PR (p= 0.114), and HER2/neu (p= 0.415). However, there was statistically significant differences between non-relapse and relapsed group in Ki-67 (p= 0.025).
The comparative analysis of the adjuvant endocrine therapy and radiotherapy after surgery of the non-relapsed and relapsed groups was summarized in Table 2. When we analyzed the effects of endocrine therapy on recurrence in 85 patients with ER or/and PR positive, there was no statistically significant relationship between endocrine therapy and recurrence (p = 0.091). When we analyzed the effects of radiotherapy on recurrence in 89 patients who underwent BCS, there was no statistically significant relationship between radiation therapy and recurrence (p= 0.531).
We have performed multivariate analysis of the factors that are suspected to be related to the recurrence of DCIS in the univariate analysis (Table 3). We could observe that Ki-67 over 14% was a statistically significant factor associated with recurrence (p= 0.021).
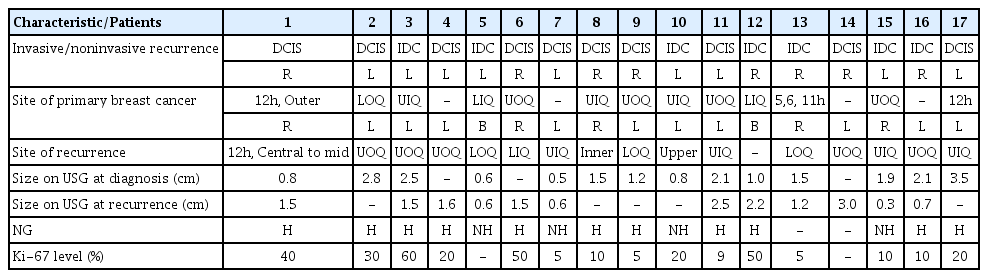
The details of 17 patients who relapsed after surgery with DCIS are shown in Table 4. In the analysis of recurrence patterns, 10 patients (58.8%) recurred as DCIS and 7 patients (41.2%) recurred as IDC. Among the 17 patients, only one patient received mastectomy after the first diagnosis of DCIS. This patient was ER positive DCIS but did not receive adjuvant hormonal therapy, and DCIS recurred in the contralateral breast. Of the 16 patients who underwent BCS surgery, 11 received radiotherapy. 11 (64.0%) had ipsilateral relapse, 4 (23.5%) had contralateral recurrence, and 2 (11.8%) had bilateral recurrence. There was no regional lymph node recurrence among the 17 patients who relapsed.
DISCUSSION
This study showed that Ki-67 could be considered as an independent prognostic factor related to DCIS recurrence. Previous largescale clinical studies have reported that postoperative adjuvant therapy, such as tamoxifen and radiation therapy, have the greatest impact on recurrence. European Organization for Research and Treatment of Cancer trial 10,853 randomized 1,010 women with surgically excised DCIS to radiation therapy (507 patients) or no radiation therapy (503 patients). They observed that the risk of any local recurrence reduced by 48% [13,14]. Our study clearly showed the limitations of small-scale retrospective studies on 104 patients with DCIS. We could not confirm the effect of adjuvant therapy such as tamoxifen or radiation therapy on reducing the recurrence rate of DCIS reported in the largescale study aforementioned.
Retrospective studies comparing clinical and pathological factors with DCIS recurrence showed various results according to their clinical setting. Choi et al. [15] reported that age and ER status were associated with recurrence in DCIS and Williams et al. [16] suggested that molecular phenotypes such as, luminal A, luminal B, HER2 type, and triple negative, and high Ki-67 expression was a significant predictor of invasive recurrence. Yun et al. [17] reviewed 431 patients with DCIS who received BCS to evaluate recurrence pattern after treatment. Among total 37 relapsed patients, 12 cases recurred as DCIS, 23 cases recurred as invasive carcinoma, and 2 cases recurred as unknown. They reported that the proportion of invasive recurrence in DCIS was 62%, which was higher than other studies. They reported that higher NG was a significant related factor of invasive recurrence.
In the univariate analysis of our study, we could see that higher NG and Ki-67 was associated with DCIS relapse. In the multivariate analysis, there was no statistically significant relation between NG and DCIS relapse, but Ki-67 was the most significant factor for predicting recurrence in patients with DCIS. A meta-analysis to examine whether Ki-67 expression can predict recurrence rates of DCIS highlights Ki-67 expression as a predictor of DCIS recurrence, aggressive disease in DCIS overall, either in situ or invasive [18]. Our result is also in line with the results of this meta-analysis in that Ki-67 plays a role as a predictive factor of recurrence.
Management strategies for DCIS vary depending on histologic grade, hormonal receptor status, and extent of disease. Almost all aspects of management for DCIS are controversial in the need for any treatment for some screen detected lesions, the extent of surgery, and the use of adjuvant endocrine or radiation therapy. Many new issues have been discussed in the DCIS area, such as the effects of low-dose tamoxifen, the need for surgery in low-grade DCIS, differential recurrence rate according to molecular subtypes or HER2-positive DCIS, and the use of oncotype Dx recurrence score to evaluate DCIS recurrence and its cost-effectiveness [16,19-21]. Recently, researchers who observed that 3rd generation aromatase inhibitor (AI) is more effective in preventing recurrence in women with postmenopausal hormone receptor-positive invasive breast cancer than tamoxifen conducted a study to see if the 3rd generation AI drug is effective in hormone receptor-positive DCIS patients. They reported that anastrozole offers another treatment option for postmenopausal women with hormone-receptor-positive DCIS, which may be more appropriate for some women with contraindications for tamoxifen [22]. These controversial issues should be confirmed through prospective large-scale clinical studies.
This study has some obvious limitations. First, this study was a small retrospective study for patients who underwent DCIS surgery in a single institute during 15 years. Therefore, the data used in this study was included a number of insufficient medical records. Second, the number of patients who recurred after DCIS treatment was so small that the factors related to recurrence of DCIS might not have obtained statistical power than its real impact on recurrence.
In conclusion, our study presented that DCIS relapse was associated higher nuclear grade and overexpressed Ki-67. Among them, Ki-67 is the most significant factor in predicting recurrence.
Notes
The authors declare that they have no competing interests.