Axillary Sampling as an Alternative Option for Complete Nodal Response in Triple Negative and HER2 Type Breast Cancer Patients after Neoadjuvant Chemotherapy
Article information
Abstract
Purpose
In patients with locally advanced breast cancer, neoadjuvant chemotherapy is widely used. It has a distinct advantage in the downstaging of the primary tumor and provides important information about treatment response. With its increasing usage, concerns over the appropriate management of the axilla have emerged. In this study, we compared oncological outcomes of conventional axillary lymph node dissection (ALND) over axillary sampling (AS) with radiotherapy (RT) in patients who received neoadjuvant chemotherapy.
Methods
In this retrospective study, we included female patients with triple negative breast cancer (TNBC) and HER2 type breast cancer who underwent breast and axillary surgery after neoadjuvant chemotherapy between May 2011 to December 2016. A total of 89 patients’ medical records were eligible for analysis. We defined AS as removal of at least four axillary lymph nodes located near the sentinel lymph nodes without full exposure of the axillary vein, long thoracic nerve, and thoracodorsal nerve.
Results
The median follow-up period was 47.00 months. The disease-free survival was 69.66 months in the AS with RT group and 69.02 months in the ALND group (p=0.280). The invasive disease-free survival was 75.16 months in the AS with RT group and 78.44 months in the ALND group (p=0.218).
Conclusion
AS with radiotherapy might be a feasible surgical option in patients with TNBC and HER2 type breast cancer after neoadjuvant chemotherapy.
INTRODUCTION
In patients with locally advanced breast cancer, neoadjuvant chemotherapy is a common practice. Neoadjuvant chemotherapy has an advantage in primary tumor downstaging and provides important information about treatment response [1,2]. After neoadjuvant chemotherapy, approximately 40%-50% of patients with clinically N1 axillary node-positive disease are converted to clinically axillary node-negative disease [3-5]. Owing to recent advances in targeted therapy, neoadjuvant chemotherapy has resulted in an increased pathologically complete response. With the increased use of neoadjuvant chemotherapy and targeted therapy, concerns over appropriate management of the axilla have emerged [6-8] due to various rates of identification of the sentinel lymph node and the substantial false-negative rate of sentinel lymph node biopsy (5%-30%). However, interpretation of these studies is controversial as surgical techniques in most of these studies have not been well standardized [9-11].
Current studies, including The American College of Surgeons Oncology Group (ACOSOG) Z1071 trial, evaluate the accuracy of sentinel lymph node biopsy after neoadjuvant chemotherapy. In this trial, the false-negative rate for patients with cN1 breast cancer after neoadjuvant chemotherapy and two or more sentinel lymph nodes examined was 12.6% [7,12,13]. The European SENTinel neoadjuvant (SENTINA) trial and the Canadian sentinel node biopsy following neoadjuvant chemotherapy (Sn FNAC) trial reported similar results [8,14]. To the best of our knowledge, few studies have compared the oncological outcomes between sentinel lymph node biopsy and axillary lymph node dissection (ALND) in patients treated with neoadjuvant chemotherapy [15]. While ALND remains the standard treatment for patients with clinically lymph node-positive disease after neoadjuvant chemotherapy, sentinel lymph node biopsy has been becoming a standard treatment for clinically lymph node-negative disease after neoadjuvant chemotherapy [6-8,16,17]. However, as proposed by researchers from the ACOSOG Z0011 trial, a less invasive axillary management strategy might be possible [3,18].
In patients with no residual disease after neoadjuvant chemotherapy, sentinel lymph node biopsy may not detect skip metastasis. Nonetheless, extensive axillary surgery might not provide better oncological benefit. However, the implementation of sentinel lymph node biopsy in patients treated with neoadjuvant chemotherapy remains a challenge. Recently, several researchers reported a new concept of axillary management, named axillary sampling (AS). AS includes limited or partial ALND; however, the concept and technique of AS are not yet clearly defined. Therefore, the clinical usefulness of AS is limited [19-22]. Nodal irradiation after sentinel lymph node biopsy has been proven as a safe alternative to ALND among patients with one or two metastatic lymph nodes undergoing surgery [23]. Rates of nodal pCR with neoadjuvant chemotherapy differ based on tumor subtype, ranging from 40% to 60% overall, and approaching 70% to 80% among patients with triple negative and HER2 type tumors [24-26]. Therefore, AS with radiotherapy (RT) could be safe for patients with triple negative and HER2 type breast cancer.
The aim of this study was to compare the oncological outcomes between conventional ALND and AS with radiotherapy in triple negative breast cancer (TNBC) and HER2 type breast cancer patients treated with neoadjuvant chemotherapy.
METHODS
Definition of ALND and AS
Conventional ALND was defined as the gross removal of most of the axillary lymph nodes with exposure of the axillary vein, long thoracic nerve, and thoracodorsal nerve. For identification of the sentinel lymph node, we used radiolabeled colloid, blue dye, or a combination of these methods. A gamma probe identified radioactivity in the lymph nodes in the axilla, and blue-stained lymphatic nodes were visually identified by surgeons. Additionally, the axilla was meticulously examined and any palpable or visually abnormal lymph nodes were resected and submitted for frozen biopsy. Clinically, AS was defined as the removal of several axillary lymph nodes located near the sentinel lymph nodes without full exposure of the axillary vein, long thoracic nerve, and thoracodorsal nerve. Additionally, based on ACOSOG Z1071 and many other clinical trials, we defined that AS should include removal of at least four lymph nodes [7,8,12-14].
Patients and surgery
In this retrospective study, we selected subjects from among 2,332 patients who had breast and axillary surgery between May 2011 to December 2016. Stage IV and clinical trial patients was excluded. Patients who received neoadjuvant chemotherapy were selected and among these, TNBC and HER2 type breast cancer patients were selected. Total of 89 patients’ medical records were eligible for analysis. These data included immunohistochemical analyses for hormonal receptor status, HER2 status, and other clinical factors, including age at the time of diagnosis, tumor size, lymph node status, and patient outcomes. After chemotherapy, axillary nodal status was re-evaluated with breast sonography and magnetic resonance imaging. This study was approved by the Institutional Review Board (IRB No. KNUCH 2019-06-012). Informed consent was waived due to the retrospective nature of the study.
Diagnosis and treatment
All cases of breast cancer were diagnosed by needle or excisional biopsy and axillary lymph node metastasis was confirmed by fine-needle aspiration cytology. The size, number, and location of the tumor were identified through mammography, ultrasonography, and breast magnetic resonance imaging prior to surgery. All patients provided written informed consent to undergo neoadjuvant chemotherapy, breast surgery, and axillary surgery. Conventional ALND included the removal of level I and II axillary lymph nodes. During AS, patients received ALND when more than four metastatic lymph nodes were detected with frozen biopsy. For ALND group, most of cases were planned ALND. Adjuvant radiotherapy was recommended to patients who received AS, and patients with pN2 after ALND were recommended to undergo adjuvant radiotherapy.
Surveillance
After surgery, all patients were followed up with radiological imaging and intensive physical examination biannually for the first two years and annually for another three years. Tumor recurrence or metastasis was evaluated with blood tests, tumor markers, mammography, breast sonography, breast magnetic resonance imaging, chest X-ray, and bone scan.
Statistical analysis
The clinical variables assessed included age, type of breast cancer, clinical and pathological tumor size, number of metastatic and removed axillary lymph nodes, overall cancer stage, and results of immunohistochemical staining. Oncological results included disease-free survival, locoregional recurrence, distant metastasis, and death.
For comparison of ALND and AS with RT, Pearson’s chi-square test or Student t-test were used, depending on the variables. Disease-free survival was defined as the time from diagnosis to the first event ending disease-free survival, including locoregional recurrence, distant metastasis, contralateral breast cancer, other primary cancer, or death from any other cause. Invasive disease-free survival was defined as the time from diagnosis to the first event showed distant metastasis or death from any other cause. Survival analysis was constructed using the Kaplan-Meier method and differences were assessed using the log-rank test. Cox regression analysis was used in the univariate and multivariate analyses. Statistical significance was accepted for a p-value of < 0.05.
RESULTS
Patient characteristics
The median follow-up period was 47.00 months (range: 9-96 months). Clinicopathologic characteristics of the patients are listed in Table 1. A total of 25 patients received AS with RT (28.1%). In both groups, clinical T, N stage, pathologic T, N stage showed no significant difference. Post neoadjuvant chemotherapy clinical N stage (ycN stage) showed no significant difference. Pathological characteristics showed no significant difference between the two groups. Neoadjuvant chemotherapy regimen showed difference between both groups. In AS with RT group, 68.0% of patients received axillary radiotherapy whereas, 14.1% of patients received axillary radiotherapy in ALND group.
Survival analysis according to types of axillary surgery
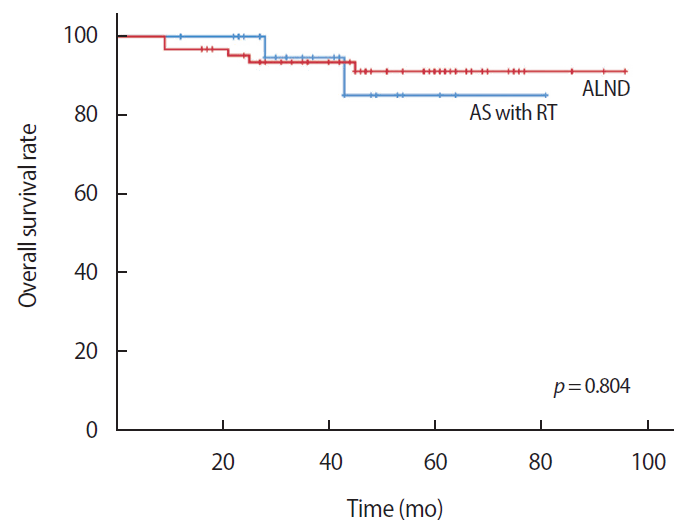
The disease-free survival was 69.66 months in the AS with RT group and 69.02 months in the ALND group. There were no statistically significant differences between the two groups (p=0.280) (Figure 1). The invasive disease-free survival was 75.16 months in the AS with RT group and 78.44 months in the ALND group. There were no statistically significant differences between the two groups (p=0.218) (Figure 2). The overall survival was 74.61 months in the AS with RT group and 89.68 months in the ALND group. There was no statistically significant difference between the two groups (p=0.804) (Figure 3).
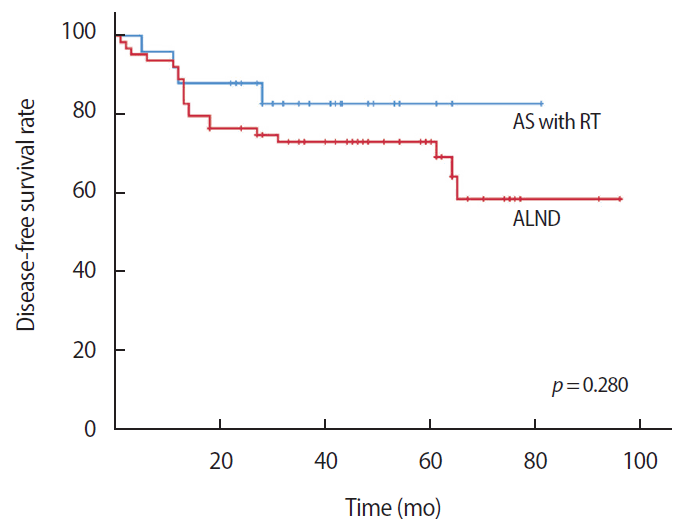
Kaplan–Meier plots for disease-free survival comparing the axillary sampling (AS) with radiotherapy (RT) group and the axillary lymph node dissection (ALND) group.
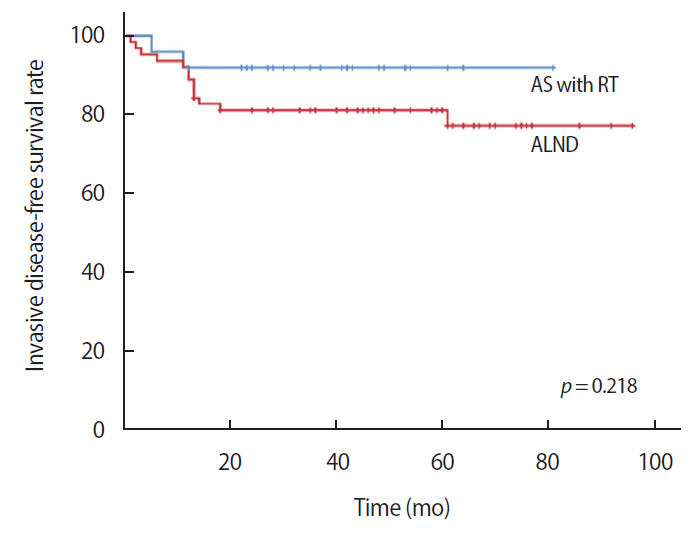
Kaplan–Meier plots for invasive disease-free survival comparing the axillary sampling (AS) with radiotherapy (RT) group and the axillary lymph node dissection (ALND) group.
Univariate and multivariate analysis for associated factors
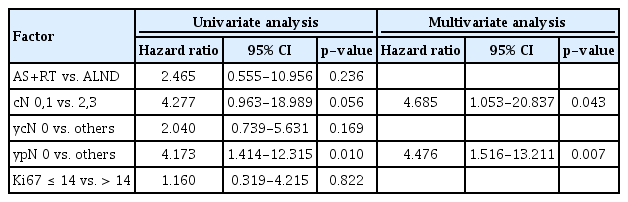
We investigated the related factor for invasive disease-free survival. In univariate analysis, post neoadjuvant chemotherapy pathologic N stage (ypN 0 vs. others, hazard ratio 4.173, p=0.010) is correlated with invasive disease-free survival. AS with RT vs. ALND exhibited no significant relationship with invasive disease free survival. In multivariate analysis, clinical N stage (cN 0,1 vs. 2,3, hazard ratio 4.685, p=0.043) and post neoadjuvant chemotherapy pathologic N stage (ypN 0 vs. others, hazard ratio 4.476, p=0.007) had strong prognostic significance for invasive disease free survival (Table 2).
DISCUSSION
Axillary lymph node dissection is an important element in the surgical management of breast cancer because it provides staging information and removes metastatic lymph nodes. However, ALND is associated with lymphedema, arm pain, sensory defects, decreased range of motion, and other morbidities that could impair quality of life. Sentinel lymph node biopsy can decrease the risk of these morbidities and has become a standard method for the evaluation of axillary lymph nodes [19-22,27]. However, ALND remains standard care for patients with a clinically positive lymph node before neoadjuvant chemotherapy.
Recently, it has been questioned whether ALND should be performed in breast cancer cases with only one to three metastatic lymph nodes. The American College of Surgeons Oncology Group (ACOSOG) Z0011 trial, the International Breast Cancer Study Group (IBCSG) 23-01 trial, and the Comparison of Complete Axillary Lymph node Dissection With Axillary Radiation Therapy in Treating Women With Invasive Breast Cancer (AMAROS) trial provided suggestions about the management of axillary lymph nodes [18,23,28,29]. Since then, many surgeons have considered less invasive techniques than ALND, including AS, sentinel lymph node dissection, or partial ALND [19-22]. Although AS could reduce the complications associated with ALND, many surgeons do not accept the concept due to lack of evidence for its oncological safety and it’s unclear definition. These issues may impact current comparison studies of AS.
We defined AS in clinical and pathological ways through a multidisciplinary team discussion. AS included dissection of the sentinel lymph nodes and other lymph nodes in proximity to the sentinel lymph nodes. In this study, we attempted to remove more than two lymph nodes, including the sentinel lymph nodes and other surrounding nodes. According to some prospective trials (ACOSOG Z1071 trial, SENTINA trial, Sn FNAC trial), removing more than two lymph nodes could reduce the false-negative rate [7,8,14]. In our results, the mean number of lymph nodes removed was 6.48± 2.83 in the AS with RT group. Using this method, the accuracy of axillary lymph node staging may be sufficiently obtained. Axillary RT field included axillary lymph node level I, II, and III. Internal mammary lymph node and supraclavicular lymph node could be included in the treatment field. This RT field definition was following ESTRO consensus guidelines [30].
The aim of this study was to compare the oncological outcomes between AS with RT and ALND with TNBC in HER2 type breast cancer after neoadjuvant chemotherapy. Our results show that diseasefree survival, invasive disease-free survival, and overall survival were not significantly different between the AS with RT group and the ALND group. Based on these results, AS with RT could satisfy oncological safety in patients with a clinically complete axillary nodal response after neoadjuvant chemotherapy.
In our study result, the axillary complete nodal response was 64.0% for all patients and was consistent with other studies [3-5]. This study indicates that at least 50% of patients who received neoadjuvant chemotherapy may not need ALND. The morbidity of axillary dissection is a great concern for both patients and clinicians; thus, the selection of appropriate patients is important. Several nomograms were developed for this purpose; [15,31] however, these nomograms require further external validation and sentinel lymph node detection methods warrant greater standardization.
Some of the strengths of our study include a reduction in the falsenegative rates associated with AS due to sentinel lymph node biopsy only. In addition, we utilized frozen biopsy samples to detect metastatic lymph nodes. Patients with more than three metastatic lymph nodes underwent ALND, reducing the possibility of under treatment.
The utilization of frozen biopsy samples to detect metastatic lymph nodes allowed us to select patients who did not need ALND. Our retrospective study has a limitation owing to the study design. However, results from this study provide evidence to support future prospective randomized controlled trials evaluating the role of AS with RT in patients who received neoadjuvant chemotherapy. Another limitation of this study was the ambiguous definition of AS. Despite our attempt at defining AS in clinical and pathological terms, a clear definition of the procedure has yet to be established.
AS with radiotherapy might be a feasible surgical option for patients with TNBC and HER2 type breast cancer after neoadjuvant chemotherapy. In addition, AS may be an alternative modality to reduce multiple morbidities caused by ALND. However, as this surgical technique should be applied carefully, the selection of appropriate patients is crucial.
Notes
The authors declare that they have no competing interests.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (2014R1A5A2009242, 2017R1C1B5076186) and by a grant from the National R&D Program for Cancer Control, Ministry of Health and Welfare, Republic of Korea (1420040). In addition, this research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI17C1142). This work was also supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2019R1A2C1006264), as well as the National Research Foundation of Korea (NRF) grants funded by the Korea government (2017M3A9G8083382), and the Foundation of Korea (NRF) grant funded by the Korea government (2019R1F1A1063853).