Breast Cancer with Delayed Metastasis Only to Contralateral Regional Lymph Nodes: A Case Report and Review of the Literature
Article information
Abstract
Delayed metastasis only to contralateral regional lymph nodes of a unilateral breast cancer has been considered as a systemic metastasis or a regional metastasis from contralateral primary occult breast cancer. This staging uncertainty has complicated for clinicians to choose treatment regimens for it. We report a case of a 52-year-old woman with metastasis only to contralateral regional lymph nodes 20 months after prior treatment of a unilateral breast cancer. She was treated with lymph node dissection, radiation therapy, chemotherapy, and hormonal therapy. Now 13 months after second surgery, the patient is alive without any evidence of breast cancer recurrence or metastasis. We suggest that solitary contralateral regional metastasis, especially post-breast-conserving surgery followed by radiation therapy, should be considered not only as hematogenous spread but also lymphatic spread. Although controversial, lymph node dissection for the delayed metastasis of breast cancer only to the contralateral regional lymph nodes can be of curative intent.
INTRODUCTION
Metastasis to the contralateral regional lymph nodes is commonly seen in the end stage of breast cancer, when metastasis has already developed elsewhere. The incidence of such cases in the literature is between 3.6% and 6% of stage IV breast cancer patients [1]. Traditionally, the contralateral regional metastasis has been regarded as distant metastasis (M1), and it is classified as such according to the American Joint Committee on Cancer staging manual [2].
But, contralateral regional lymph node metastasis alone as a recurrence of a unilateral breast cancer treated before is uncommon. The presentation of breast cancer cell only in the contralateral regional lymph node following prior treatment of a unilateral breast cancer can be considered as a systemic disease, on second thought, it can be a regional metastasis from contralateral primary occult breast cancer.
We report a case of metastasis only to contralateral regional lymph nodes that arose 20 months after a unilateral breast cancer treated with breast-conserving surgery, radiation therapy, and adjuvant chemotherapy. To follow the traditional principle, contralateral regional metastasis is systemic disease, and it should be treated with systemic therapy, not with local control [3]. But, by reviewing the related literature, we got to know that the contralateral regional metastasis can be mainly due to lymphatic spread [1,4,5], so we intend to suggest an alternative way to approach contralateral regional metastasis.
CASE REPORT
A 52-year-old postmenopausal woman presented to the outpatient breast cancer clinic of our hospital because of a palpable mass in her right breast in August 2010. Mammography and ultrasonography revealed a tumor mass measured 2.6× 2.0 cm in upper inner quadrant of her right breast without evidence of axillary lymphadenopathy. We performed an ultrasonographic-guided core biopsy of the mass in the right breast, and histopathological examination of the biopsy specimen revealed poorly differentiated invasive carcinoma. Breast magnetic resonance imaging (MRI), computed tomography (CT) of the chest and abdomen and bone scan showed no evidence of metastatic disease outside of the right breast mass.
The patient underwent breast-conserving surgery and axillary sentinel lymph node biopsy. Tissue from the right breast contained a grade II invasive ductal carcinoma measured 4.0×3.0 cm, with negative margins (Figure 1). Immunohistochemistry showed the following signs: estrogen receptor (ER) (-), progesterone receptor (PR) (-), c-erbB-2 (2+), CK5/6 (-), E-cadherin (+), p53 (+), and Ki-67 index 15%. Reanalyzing by fluorescence in situ hybridization showed the tumor was negative for c-erbB-2 amplification. There was no evidence of carcinoma in eight resected axillary lymph nodes. Following surgery, the patient received adjuvant radiation therapy, which delivered a total dose of 58 Gy in 32 fractions to the whole right breast. She received additional six cycles of adjuvant chemotherapy with cyclophosphamide, methotrexate, and 5-fluorouracil until February 2011. Since then, the patient was followed up with every 3 months of clinical examination and every 6 months of imaging workup.
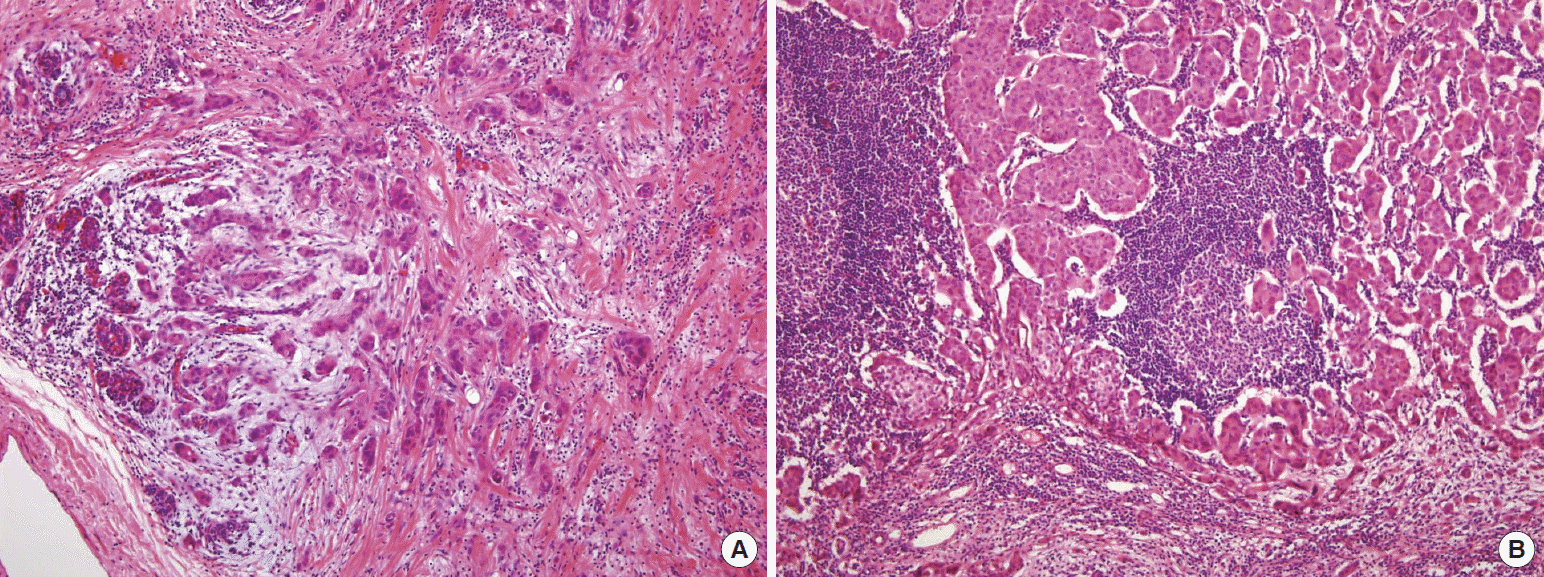
Microscopic findings. (A) Right breast specimen revealed clusters of infiltrating ductal carcinoma (H&E stain, ×200). (B) Left axillary lymph node specimen revealed clusters of infiltrating ductal carcinoma (H&E stain, ×200).
Twenty months after surgery, in April 2012, ultrasonography showed the presence of enlarged lymph nodes in left axilla and left supraclavicular area. Therefore, fine-needle aspiration cytology of the left axillary lymph node was obtained, which contained metastatic carcinoma. There was not any suspicious lesion on the bilateral breast and no evidence of metastasis except left axilla and supraclavicular area in mammography, ultrasonography, CT, bone scan, and torso positron emission tomography (PET) (Figure 2).
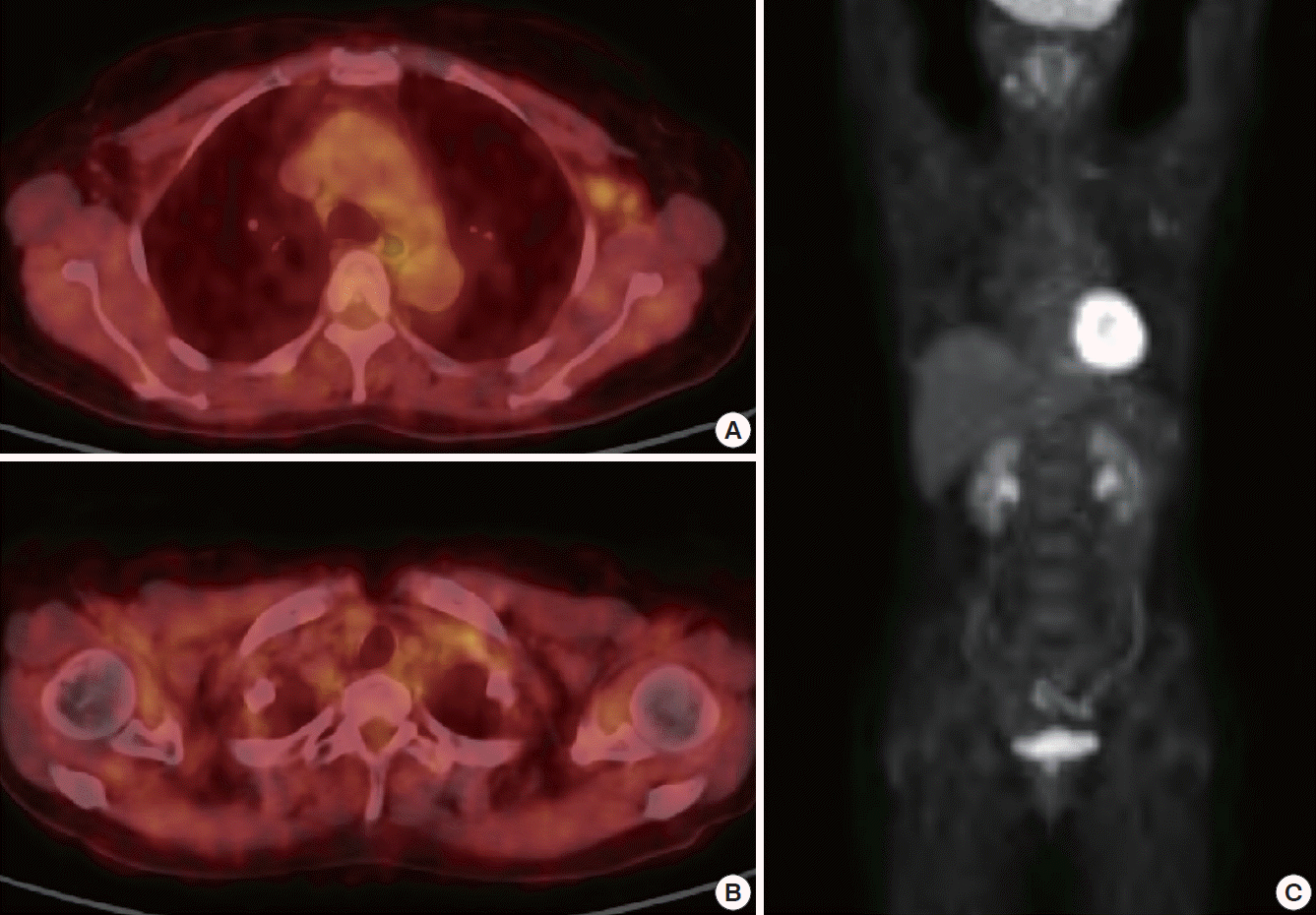
The torso positron emission tomography scan, showing contralateral axilla metastasis (maximum standardized uptake value [SUVmax]=2.11) (A) and contralateral supraclavicular metastasis (SUVmax=2.22) (B) without any other metastasis (C).
Complete left axillary and supraclavicular lymph node dissection was performed in May 2012. Histopathologically, 54 of 71 lymph nodes contained metastatic invasive ductal carcinoma, which included 26 of 38 axillary lymph nodes and 28 of 33 supraclavicular lymph nodes. Immunohistochemical analysis showed the following signs: estrogen receptor (+), progesterone receptor (-), c-erbB-2 (2+), CK5/6 (-), E-cadherin (+), p53 (+), and Ki-67 index 15%. Reanalyzing by silver in situ hybridization showed the tumor was negative for c-erbB-2 amplification. Following the second surgery, the patient subsequently underwent four cycles of doxorubicin and cyclophosphamide chemotherapy and four cycles of docetaxel chemotherapy until November 2012. And then, she received additional radiation therapy on left axilla and supraclavicular fossa, and the dose delivered was a total of 50 Gy in 28 fractions. Tamoxifen (20 mg daily) was added to her systemic therapy due to the new identification of estrogen receptor.
For the last 13 months after the second surgery, the patient has been followed up with mammography, ultrasonography, CT, bone scan, and torso PET, and there was no clinical evidence of tumor recurrence or metastasis in the latest work-up of May 2013.
DISCUSSION
When lymph node metastasis from an undefined primary tumor is diagnosis, accurate decision of primary tumor site is very important, because the therapeutic approaches can vary depending on the primary tumor [6]. So, the patient with the axillary lymph node metastasis of unknown origin should undergo whole body imaging work-up like CT, bone scan, and PET [7]. Since breast cancer is the most common cause of axillary metastasis, breast specific examination like mammography and breast ultrasonography should be included in the imaging work-up [8]. Breast MRI is considered to be superior to mammography or ultrasonography in sensitivity, but it is controversial whether MRI is helpful to detect the occult primary tumor from the breast [7,9,10]. A careful histopathological examination for the metastatic lymph nodes is essential to specify the primary tumor site, and especially immunohistochemistry like ER, PR, and human epidermal growth factor receptor 2 (HER2) can help to determine whether the metastasis originated from breast cancer or not [11].
The breast-originated cancer cell only in axillary or supraclavicular lymph node may be the evidence of regional metastasis from an occult primary breast cancer. But, in the patients having been treated for breast cancer, it is more likely to be metastatic lesions of the breast cancer treated before.
Because contralateral spread of crossed the midline of a unilateral cancer implies hematologic spread, axillary lymph nodes metastasized from the opposite breast cancer have been regarded as an evidence of systemic disease [3]. But, by reviewing the related literature, we came to understand that the contralateral regional lymph node metastasis can be thought of not only as hematogenous spread but also lymphatic spread [1,4,5]. Blockage of or damage to the usual lymphatic system might lead to the development of alternative routes of drainage. This blockage can happen because of surgery, radiotherapy or even tumor cells in the lymphatics. This has been shown in many studies comparing lymphatic drainage of the breast before and after breast surgery for cancer, which showed that deformed lymphatic drainage is common after surgery [4,12].
It is important to perceive that contralateral regional metastasis occurs mainly by lymphatic spread in most patients, and not by hematogenous spread [4]. This is supported by many experimental studies [4,12]. It is also partially supported by the fact that not a few of the patients have contralateral regional metastasis without systemic metastasis.
Management of patients with contralateral regional metastasis is not straightforward, especially with the absence of metastatic disease elsewhere. Treatment probably should be individualized. If contralateral regional metastasis accompanies systemic metastasis, the systemic treatment should be essential. Surgery to the metastatic lymph nodes can be performed in some cases for local control and palliation. In patients with contralateral regional lymph node metastasis as the only site outside the breast, therapeutic options are more controversial. Contralateral axillary dissection can be one good option, which results in excellent axillary control [4]. The patient of this study, who underwent axillary and supraclavicular dissection, has not had any recurrent sign for the last 13 months after the second surgery up to the latest work-up of May 2013. Radiation therapy can give an additional help to the local control. Routine contralateral mastectomy is probably not indicated [1]. Mastectomy might be considered in some cases of primary lobular carcinoma. Hereditary breast cancer is another possible indication for performing mastectomy.
Of course, systemic therapy should be included in the treatment of contralateral regional metastasis. Hormonal therapy would be the first option in patients whose tumors are hormone receptor positive. Undoubtedly, systemic chemotherapy should be performed because of the possibility of coexistence of the other metastasis. Trastuzumab will do better to being added if there is HER2 overexpression.
In conclusion, we suggest that contralateral regional lymph node metastasis, especially after breast-conserving surgery following radiation therapy, should be considered not only as hematogenous spread but also lymphatic spread. If there is no evidence of other metastatic lesions, lymph node dissection needs to be carried out, and radiation therapy can be added following proper systemic therapy. Although strong evidence is lacking, it can be of curative intent.
Notes
CONFLICT OF INTEREST
The authors declare that they have no competing interests.