AbstractPurposeAccording to American Society of Clinical Oncology/College of American Pathologists guidelines, breast cancer is human epidermal growth factor receptor 2 (HER2) positive if there is HER2 protein overexpression at a 3+ level on immunohistochemistry (IHC 3+) or gene amplification (more than six copies per nucleus) on fluorescence in situ hybridization (FISH+). However, there have been few reports on whether outcomes differ based on diagnosis by these two techniques. In this study, we compared outcomes based on the two methods in patients with HER2-positive breast cancer.
MethodsThis study was a retrospective analysis of HER2-positive breast cancer in 18,304 patients, including 14,652 IHC 3+ patients and 3,652 FISH+ patients from the Korean Breast Cancer Society Registry. We compared breast cancer-specific survival and overall survival based on IHC 3+ and FISH+ status with or without trastuzumab.
ResultsBreast cancer-specific survival was significantly different between the IHC 3+ and FISH+ groups, with 5-year cumulative survival rates of 95.0% for IHC 3+ and 98.5% for FISH+ patients who did not receive trastuzumab (p=0.001) in Kaplan-Meier methods. However, there were no significant differences in breast cancer-specific survival and overall survival between IHC 3+ and FISH+ groups regardless of trastuzumab treatment in Cox proportional hazards models.
INTRODUCTIONHuman epidermal growth factor receptor 2 (HER2), a transmembrane receptor tyrosine kinase that is encoded on the long arm of chromosome 17 (17q12-21.32), is involved in tumor growth and progression. Overexpression of HER2 and gene amplification are displayed in 15% to 20% of breast cancer patients and are associated with aggressive cancer and a poor prognosis [1-4].
Trastuzumab, a representative targeted therapeutic agent for HER2-positive breast cancer, acts to arrest the cell cycle G1 phase and blocks cell proliferation with a humanized monoclonal antibody attached to the extracellular domain of the HER2 receptor. The drug, approved by the Food and Drug Administration (FDA) in 1998, has helped improve survival outcomes in both adjuvant and metastatic settings for HER2-positive breast cancer [5-8]. Clinical trials, such as NSABP B-31 and NCCTG N9831, have shown that the addition of trastuzumab in an adjuvant setting increases disease-free survival [9]. Since such HER2-targeted therapies are only indicated for HER2-positive breast cancer, identification of HER2 positivity is important [10].
HER2 positivity is generally determined by immunohistochemistry (IHC), which measures the degree of overexpression of the HER2 protein. Although IHC is inexpensive and easy to perform, HER2 positivity may often be difficult to conclusively determine due to specimen heterogeneity and reader subjectivity in the interpretation of HER2 protein expression level [11]. The HER2 in situ hybridization (ISH) method, which measures HER2/neu gene amplification, is used in cases equivocal by IHC. ISH encompasses fluorescence ISH (FISH), chromogenic ISH, silver ISH (SISH), and dual color ISH [7]. FISH, in particular, has excellent sensitivity and specificity and is used as a golden standard in this setting [10].
According to American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) guidelines, an IHC score of 3+ or gene amplification of more than six copies per nucleus by ISH qualifies as HER2 positive [6]. Although HER2 protein overexpression and HER2/neu gene amplification are closely related to each other, a debate remains on whether there is a difference in prognosis between the two for diagnosing HER2 cancer [12,13]. The aim of this study was to compare prognosis between the two methods in HER2-positive breast cancer patients.
METHODSIn this study, we used materials approved by the Korean Breast Cancer Society registration system (KBCR). KBCR has been registering cases submitted by breast surgeons at 110 training hospitals nationwide since 1996 and has previously been described in detail [14-17]. The cause and date of death in the documents were used in connection with the Korea Central Cancer Registration Data of the Ministry of Health and Welfare in collaboration with the Korean National Statistical Office to compile complete death statistics, which were updated through 2014. This study was approved by Daejeon St. Mary’s Hospital Institutional Review Board (IRB; No. DC16OSSI0071). The IRB approving the study did not require additional informed consent to be obtained. Of the 122,126 patients diagnosed with breast cancer from June 1983 to December 2015, 18,304 patients were included in this study after others were excluded as described in Figure 1. Of these, 14,652 patients treated without trastuzumab and 3,652 patients treated with trastuzumab were retrospectively analyzed in the 3+ group by IHC test and the HER2/neu gene amplification group by FISH. Based on the criteria for a HER2-positive diagnosis, each patient was classified as IHC 3+ and FISH+ (Figure 1). For prognostic measurements, based on the presence or absence of trastuzumab, we compared breast cancer-specific survival and overall survival between IHC 3+ and FISH+ groups, and we further analyzed factors affecting survival rate.
ImmunohistochemistryIHC follows a 4-step scoring system from 0 to 3+, which depends on the immunostaining intensity in the cell membrane of the tissue specimen, as a way to measure the amount of HER2 protein in paraffin sections of breast cancer tissue. According to ASCO/CAP guidelines, specimens are classified as 0 to absent, 1+ to faint, 2+ to weak, and 3+ to intense from more than 10% cells [18]. A score of 3+ is associated with HER2/neu gene amplification in almost all cases, and IHC alone determines HER2 positivity.
Fluorescence in situ hybridization assayThe HER2 gene is located on chromosome 17, and gene amplification is measured by displaying it. When more than six copies are shown per nucleus or when the HER2/CEP17 ratio is higher than 2.0, the sample is considered FISH+ for HER2. FISH as a predictor of response to HER2 target therapy is more sensitive and specific than IHC. Further, when IHC is equivocal (IHC 2+), FISH is used to determine HER2 positivity.
Description of the study cohortPatient baseline clinical characteristics included age, family history, surgical method, pathological TNM stage, menopause status, diagnostic method for HER2 positive (IHC 3+ or FISH+), histologic grade, nuclear grade, and adjuvant therapies, such as chemotherapy, radiotherapy, and endocrine therapy.
Statistical analysisPrognosis was compared using the SAS System for Windows version 9.3 (SAS Institute Inc., Cary, USA). Comparisons of baseline characteristics were performed using the Student t-test, Pearson chisquare test, or Fisher exact test. The correlation between IHC and FISH and other clinical factors was compared by logistic regression analysis, and comparisons of survival rates were performed with Kaplan-Meier methods and Cox proportional hazard models.
RESULTSClinicopathological characteristicsThe median follow-up period was 65 months (range, 0–234 months) in the 18,304 patients. The proportion of patients who used trastuzumab was lower in IHC 3+ patients (18.7%) compared to FISH+ (34.5%) patients (p< 0.001). To consider possible prognostic differences due to trastuzumab, IHC 3+ and FISH+ patients were compared after stratification by trastuzumab treatment. Basic clinicopathological characteristics and survival were compared between patients with or without trastuzumab treatment and described as Table 1. The average and standard deviation of age was 50.21±10.38 years for IHC 3+ and 51.92±11.15 years for FISH+ patients who did not receive trastuzumab (p< 0.001), and 50.72± 9.50 years for IHC 3+ and 51.39±10.55 years for FISH+ patients who received trastuzumab (p = 0.181).
Clinicopathological factors associated with HER2 positive breast cancer according to diagnostic methodsBased on univariate logistic regression analysis in patients who did not receive trastuzumab, FISH+ was more associated with age, nuclear grade (grade 2–3), radiotherapy, and endocrine therapy, while IHC 3+ was more associated with mastectomy, axillary dissection, pathologic stage (stage III), premenopause, histologic grade (grade 2–3), negative hormonal receptor status, breast cancer-specific survival, and overall survival. In patients who received trastuzumab, FISH+ was more associated with family history, nuclear grade (grade 2), radiotherapy, and endocrine therapy, and IHC 3+ was more associated with mastectomy, axillary dissection, pathologic stage (stage III), negative hormonal receptor status, and overall survival (Table 2). In multivariate analysis, the breast cancer-specific survival and overall survival factors were analyzed separately. For breast cancer-specific survival and patients who did not receive trastuzumab, FISH+ was more associated with age, pathologic stage (stage II–IV), nuclear grade (grade 2–3), radiotherapy, and chemotherapy, and IHC 3+ was more associated with axillary dissection, histologic grade (grade 2–3), negative hormonal receptor status, endocrine therapy, and breast cancer-specific survival. In patients who received trastuzumab, FISH+ was more associated with nuclear grade (grade 2–3), and IHC 3+ was more associated with axillary dissection and negative hormonal receptor status (Table 3). For overall survival and patients who did not receive trastuzumab, FISH+ was more associated with age, pathologic stage (stage II–IV), nuclear grade (grade 2–3), radiotherapy, and chemotherapy, and IHC 3+ was more associated with axillary dissection, histologic grade (grade 2–3), negative hormonal receptor status, endocrine therapy, and overall survival. In patients who received trastuzumab, FISH+ was more associated with nuclear grade (grade 2–3), and IHC 3+ was more associated with axillary dissection and negative hormonal receptor status (Table 4).
Survival comparisonsIn survival comparisons using the Kaplan-Meier method, 5-year cumulative survival rates of the whole HER2 subtype cohort were 95.7% and 90.8% for breast cancer-specific survival and overall survival, respectively. According to the presence or absence of trastuzumab treatment, 5-year cumulative survival rates were 99.1% and 95.2% for breast cancer-specific survival and 94.3% and 90.3% for overall survival, respectively. The HER2 subtype cohort was significantly different according to the pathologic stage in both breast cancer-specific survival and overall survival (Figure 2).
Breast cancer-specific survival was significantly different between IHC 3+ and FISH+ patients. In patients who did not receive trastuzumab, 5-year cumulative survival rates were 95.0% for IHC 3+ and 98.5% for FISH+ patients (p = 0.001). In patients who received trastuzumab, 5-year cumulative survival rates were 99.1% for IHC 3+ and 100.0% for FISH+ (p = 0.292). There was no difference in overall survival rates between the IHC 3+ and FISH+ groups, either with or without trastuzumab treatment (Figure 3). In Cox proportional hazard models, depending on the presence or absence of trastuzumab use, the factors affecting breast cancer-specific survival were age (hazard ratio [HR], 1.023, p = 0.011), mastectomy (HR, 1.989, p< 0.001), axillary dissection (HR, 4.006, p< 0.001), pathologic stage (stage II: HR, 2.648, p< 0.001; stage III: HR, 6.753, p< 0.001; stage IV: HR, 14.921, p < 0.001), histologic grade (grade 3: HR, 2.053, p = 0.041), nuclear grade (grade 2: HR, 0.534, p = 0.012; grade 3: HR, 0.598, p = 0.029), and chemotherapy (HR, 0.586, p = 0.007) in patients who did not receive trastuzumab, and none in patients who received trastuzumab (Table 5). Factors affecting overall survival were mastectomy (HR, 1.653, p< 0.001), axillary dissection (HR, 1.838, p< 0.001), pathologic stage (stage II: HR, 2.543, p< 0.001; stage III: HR, 6.159, p< 0.001; stage IV: HR, 13.390, p< 0.001), histologic grade (grade 2: HR, 1.518, p = 0.038; grade 3: HR, 1.816, p = 0.004), and chemotherapy (HR, 0.490, p< 0.001) in patients who did not receive trastuzumab, while pathologic stage (stage III: HR, 6.012, p = 0.004; stage IV: HR, 228.381, p< 0.001), negative hormonal receptor status (HR, 3.169, p = 0.005), and endocrine therapy (HR, 2.216, p = 0.040) affected overall survival in patients who received trastuzumab (Table 6).
DISCUSSIONImplicated in the growth of breast cancer, the HER2 receptor has become a target for breast cancer therapy. Since the discovery of the HER2/neu oncogene, FISH has helped overcome the diagnostic ambiguity of the existing IHC method for determining HER2 positivity [19,20]. HER2 gene amplification in FISH is more than 90% identical to HER2 receptor protein overexpression, and HER2 positivity is related to prognosis. However, in some cases, there may be overexpression of the HER2 receptor without HER2 gene amplification, and vice versa, leading to inconsistent results [21]. Although FISH is used as a gold standard for measuring HER2/neu gene amplification, SISH has been shown to be as feasible as FISH to determine HER2 status in some recent studies [22].
Correlations between these two diagnostic methods in HER2-positive breast cancer differ from study to study. Petroni et al. [12] reported that HER2-positive breast cancer showed unfavorable features in patients exhibiting gene amplification in FISH, even though the HER2 protein was expressed at lower amounts. Mass et al. [23] analyzed three clinical trials targeting overexpression in IHC 2+ or 3+ patients, and they reported that treatment of metastatic breast cancer with trastuzumab in FISH-positive patients improved response and survival rates compared to FISH-negative cancer, indicating that FISH can be an important method for diagnosis of HER2 positivity. In contrast, Toi et al. [24] reported that in patients with metastatic HER2-positive breast cancer, overall survival was significantly higher in the group with a higher degree of HER2 expression, and the effects of trastuzumab could be seen. Lipton et al. [25] reported that most HER2 IHC 3+ cases were concordant with positive FISH in 102 patients with metastatic breast cancer, and higher levels of gene amplification in FISH and HER2 protein expression in IHC were associated with a relatively longer time to progression. Additionally, FISH positive cases with low HER2 protein expression have a similar prognosis to FISH negative cases with low HER2 protein expression.
There are also studies where the results of these two methods do not vary. Zabaglo et al. [13] reported that in the Herceptin Adjuvant (HERA) trial of early-stage breast cancer patients, HER2 staining intensity and FISH amplification had a positive correlation, independent of the degree of HER2 staining intensity; however, disease-free survival was similar in both the presence and absence of trastuzumab. Xu et al. [26] reported that the HER2 amplification level was not an effective prognostic factor, finding no significant difference in disease-free survival based on a meta-analysis of trastuzumab use in HER2 positive patients.
We observed a difference between the IHC 3+ and FISH+ groups in relation to other clinical factors. When analyzed according to the presence or absence of trastuzumab, FISH+ was mainly associated with nuclear grade, and IHC 3+ was mainly associated with axillary dissection and negative hormonal receptor status. Nuclear grade is associated with chromosome 17 polysomy in relation to FISH positivity. Chibon et al. [27] compared ASCO/CAP and FDA guidelines, finding that the HER2/CEP17 ratio was associated with mitotic count and nuclear atypia. In relation to survival factors, although IHC 3+ group was more associated with breast cancer-specific survival and overall survival than FISH+ group and a worse survival curve of breast cancer-specific survival in the absence of trastuzumab, survival differences between the two groups were not significant in Cox proportional hazard models. It is thought that other factors associated with IHC 3+ or FISH+ probably affected survival as confounding factors. Further evaluation is needed for clinical factors, such as histologic subtypes, not included in the existing analysis.
In this study, the usage rate of trastuzumab was lowered to 18.7% in the IHC 3+ group and 34.5% in the FISH group. The reasons for the low usage rates are the high proportion of early breast cancer in this study and the possibility of missing data, which is an inborn limitation of retrospective analyses. In South Korea, trastuzumab has been approved since 2003. Approval criteria are lymph node positive or tumor size greater than 1 cm. The difference in use of trastuzumab before and after the approval was 0.3% versus 21.8% in the IHC 3+ group and 0% versus 34.6% in the FISH+ group.
This study does have several limitations, including the retrospective design. Although we used large-scale data, such data were passively obtained from medical records at multiple medical institutions, leading to heterogeneity and missing data. Further, only the presence or absence of HER2 amplification in FISH using the input system could be identified, and specific criteria, such as the HER2/CEP17 ratio, could not be confirmed. Recently, there have been studies in which intrinsic subtype division using gene expression assay (such as PAM50) may be useful for therapeutic response and prognosis prediction [28]. In a typical diagnostic process for HER2, most patients do not require FISH if they have an IHC score of 3+ without FISH. Most patients with HER2 FISH assay results are patients whose breast cancers have HER2 IHC 2+ immunostaining. These patients typically have breast cancers composed of a majority of HER2-not-amplified (“FISH-negative”) breast cancers and a minority that are HER2-amplified (“FISH-positive”) breast cancers. Characteristically, only modest levels of HER2 gene amplification are associated with IHC 2+ breast cancers. In contrast, the IHC 3+ breast cancers typically have higher levels of HER2 gene amplification. In comparison, relatively few of these IHC 3+ breast cancers are expected to have modest levels of HER2 gene amplification [29,30]. It is thought that additional relevant studies are required under equivalent conditions. In addition, interpretation of IHC staining by pathologists has a subjective element, potentially introducing selection bias. Finally, there were few breast cancer-specific deaths in our cohort, making it difficult to compare detailed survival rates.
In this study, the breast cancer-specific survival and overall survival were not affected by the different two diagnostic methods of HER2-positive breast cancer in Cox proportional hazard models. A strength of this study is that we objectively evaluated no difference in survival outcome between the diagnostic methods using a large-scale dataset to overcome the limitations of previous controversial studies, such as small sample size. Further research to evaluate differences in prognosis and other characteristics according to the diagnostic methods of HER2 positivity is needed in the future.
ACKNOWLEDGMENTSThis article was supported by the Korean Breast Cancer Society, which provided data.
Figure 1.Selection and categorization of human epidermal growth factor receptor 2 (HER2)-positive patient study cohort.
IHC=immunohistochemistry; FISH=fluorescence in situ hybridization.
Figure 2.The breast cancer-specific survival and overall survival curves of the human epidermal growth factor receptor 2 (HER2) subtype cohort. (A) Breast cancer-specific survival of the whole HER2 subtype cohort. (B) Overall survival of the whole HER2 subtype cohort. (C) Breast cancer-specific survival based on trastuzumab treatment. (D) Overall survival based on trastuzumab treatment. (E) Breast cancer-specific survival according to pathologic stage. (F) Overall survival according to pathologic stage. 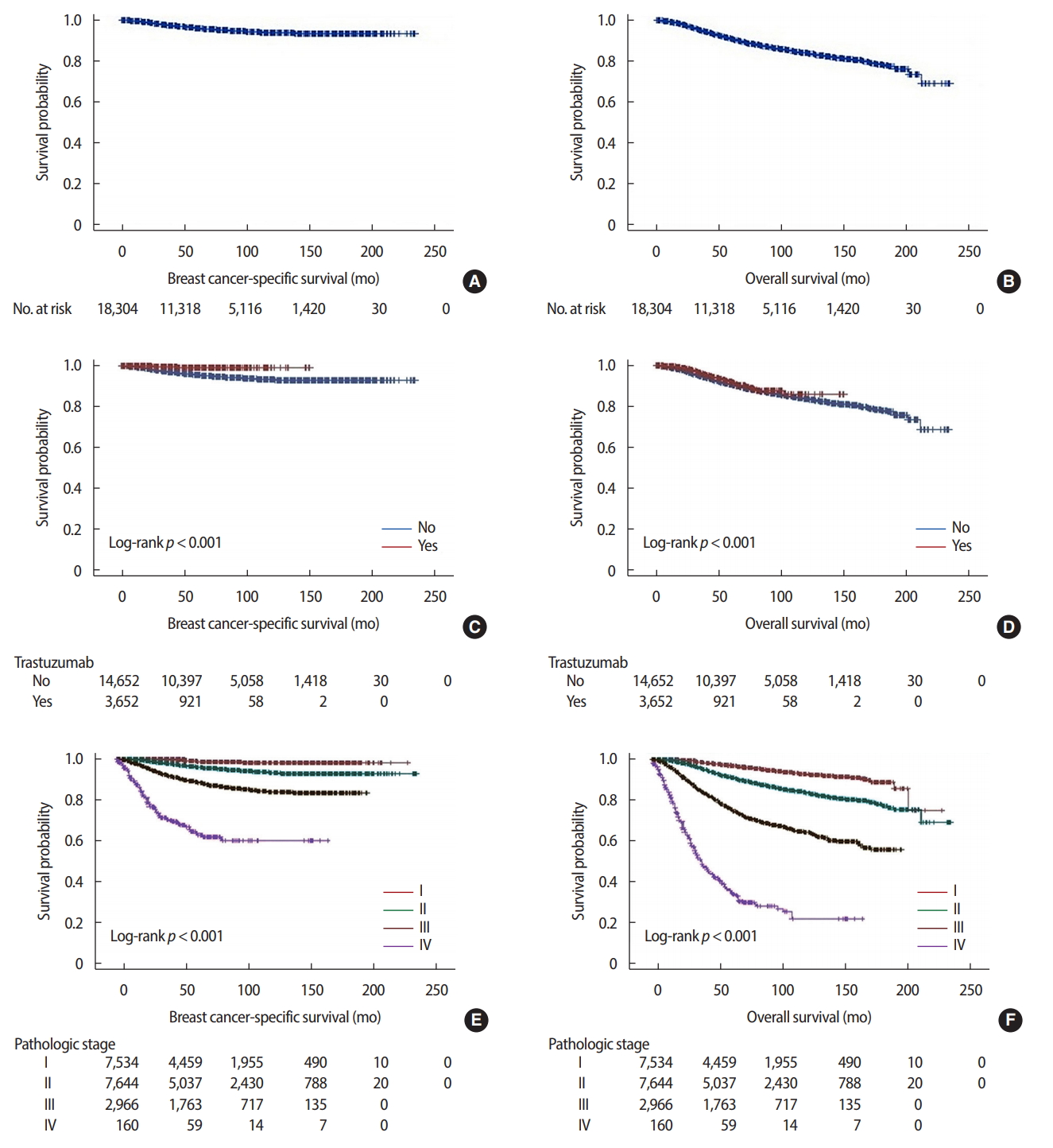
Figure 3.The breast cancer-specific survival and overall survival curves between the immunohistochemistry (IHC) 3+ and fluorescence in situ hybridization (FISH)+ groups. (A) Breast cancer-specific survival without trastuzumab. (B) Overall survival without trastuzumab. (C) Breast cancer-specific survival with trastuzumab. (D) Overall survival with trastuzumab. 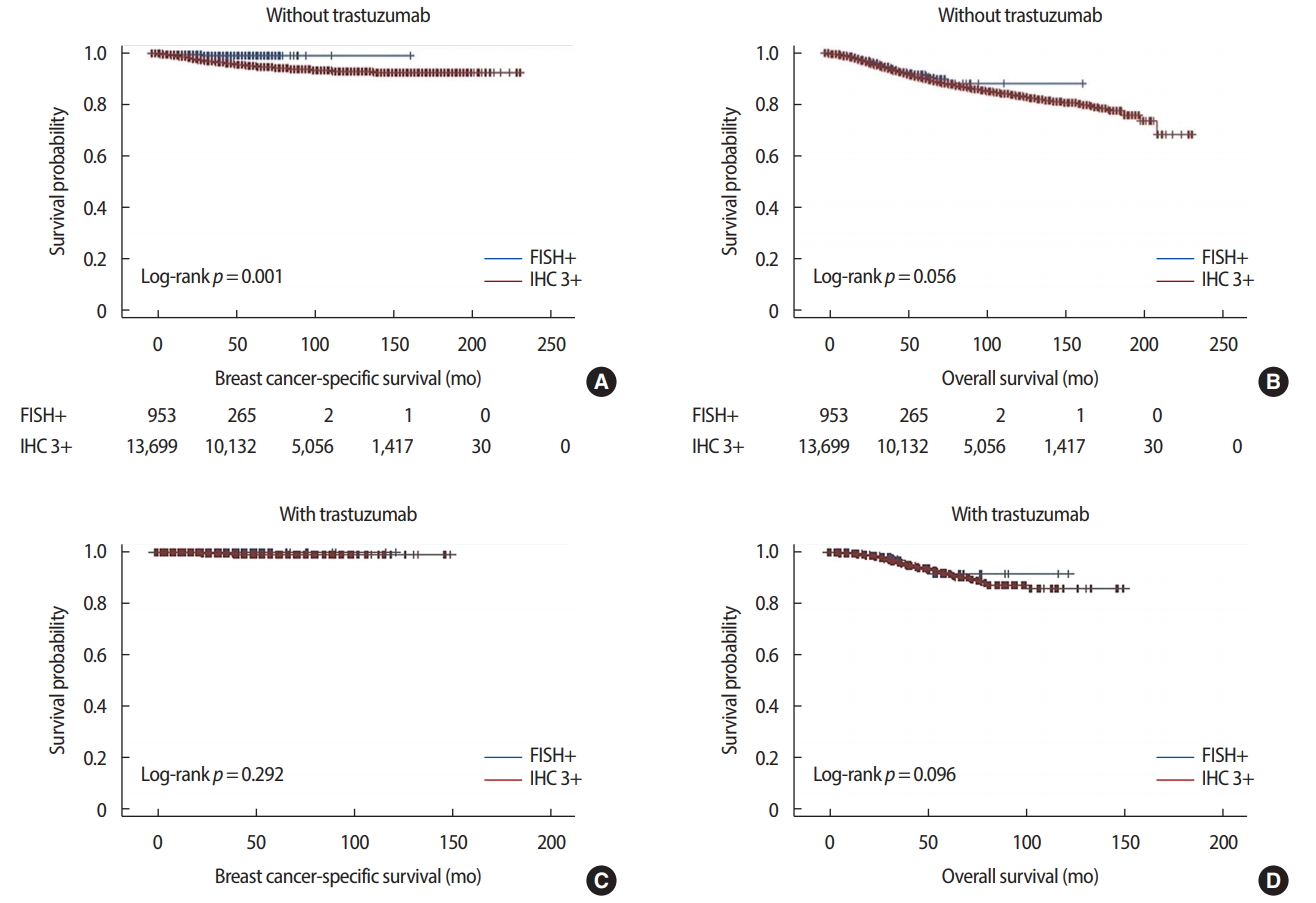
Table 1.Clinical characteristics of IHC 3+ and FISH+ whether with or without trastuzumab
Table 2.Univariate analysis for clinicopathological factors associated with IHC 3+ or FISH+ Table 3.Multivariate analysis for clinicopathological factors containing breast cancer-specific survival associated with IHC 3+ or FISH+ Table 4.Multivariate analysis for clinicopathological factors containing overall survival associated with IHC 3+ or FISH+ Table 5.Analysis of breast cancer-specific survival of the HER2-positive breast cancer patients Table 6.Analysis of overall survival of the HER2-positive breast cancer patients REFERENCES1. Ballinger TJ, Sanders ME, Abramson VG. Current HER2 testing recommendations and clinical relevance as a predictor of response to targeted therapy. Clin Breast Cancer 2015;15:171-80.
2. Christgen M, van Luttikhuizen, Raap M, Braubach P, Schmidt L, Jonigk D, et al. Precise ERBB2 copy number assessment in breast cancer by means of molecular inversion probe array analysis. Oncotarget 2016;7:82733-40.
3. Stocker A, Hilbers ML, Gauthier C, Grogg J, Kullak-Ublick GA, Seifert B, et al. HER2/CEP17 ratios and clinical outcome in HER2-positive early breast cancer undergoing trastuzumab-containing therapy. PLoS One 2016;11:e0159176.
4. Kim HA, Kim EK, Kim MS, Yu JH, Lee MR, Lee HK, et al. Association of human epidermal growth factor receptor 2 with radiotherapy resistance in patients with T1N0M0 breast cancer. J Breast Cancer 2013;16:266-73.
5. Andrulis IL, Bull SB, Blackstein ME, Sutherland D, Mak C, Sidlofsky S, et al. neu/erbB-2 amplification identifies a poor-prognosis group of women with node-negative breast cancer. Toronto Breast Cancer Study Group. J Clin Oncol 1998;16:1340-9.
6. Lim TH, Lim AS, Thike AA, Tien SL, Tan PH. Implications of the updated 2013 American Society of Clinical Oncology/College of American Pathologists guideline recommendations on human epidermal growth factor receptor 2 gene testing using immunohistochemistry and fluorescence in situ hybridization for breast cancer. Arch Pathol Lab Med 2016;140:140-7.
7. Kurozumi S, Padilla M, Kurosumi M, Matsumoto H, Inoue K, Horiguchi J, et al. HER2 intratumoral heterogeneity analyses by concurrent HER2 gene and protein assessment for the prognosis of HER2 negative invasive breast cancer patients. Breast Cancer Res Treat 2016;158:99-111.
8. Engel RH, Kaklamani VG. HER2-positive breast cancer: current and future treatment strategies. Drugs 2007;67:1329-41.
9. Perez EA, Romond EH, Suman VJ, Jeong JH, Davidson NE, Geyer CE, et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol 2011;29:3366-73.
10. Park K, Han S, Kim JY, Kim HJ, Kwon JE, Gwak G. Silver-enhanced in situ hybridization as an alternative to fluorescence in situ hybridization for assaying HER2 amplification in clinical breast cancer. J Breast Cancer 2011;14:276-82.
11. Wesoła M, Jeleń M. A comparison of IHC and FISH cytogenetic methods in the evaluation of HER2 status in breast cancer. Adv Clin Exp Med 2015;24:899-903.
12. Petroni S, Caldarola L, Scamarcio R, Giotta F, Latorre A, Mangia A, et al. FISH testing of HER2 immunohistochemistry 1+ invasive breast cancer with unfavorable characteristics. Oncol Lett 2016;12:3115-22.
13. Zabaglo L, Stoss O, Rüschoff J, Zielinski D, Salter J, Arfi M, et al. HER2 staining intensity in HER2-positive disease: relationship with FISH amplification and clinical outcome in the HERA trial of adjuvant trastuzumab. Ann Oncol 2013;24:2761-6.
14. Kim Z, Min SY, Yoon CS, Jung KW, Ko BS, Kang E, et al. The basic facts of Korean breast cancer in 2012: results from a nationwide survey and breast cancer registry database. J Breast Cancer 2015;18:103-11.
15. Min SY, Kim Z, Hur MH, Yoon CS, Park EH, Jung KW, et al. The basic facts of Korean breast cancer in 2013: results of a nationwide survey and breast cancer registry database. J Breast Cancer 2016;19:1-7.
16. Choi MY, Lee SK, Lee JE, Park HS, Lim ST, Jung Y, et al. Characterization of Korean male breast cancer using an online nationwide breast-cancer database: matched-pair analysis of patients with female breast cancer. Medicine (Baltimore) 2016;95:e3299.
17. Chang JS, Choi JE, Park MH, Jung SH, Choi BO, Park HS, et al. Trends in the application of postmastectomy radiotherapy for breast cancer with 1 to 3 positive axillary nodes and tumors ≤5 cm in the modern treatment era: a retrospective Korean Breast Cancer Society report. Medicine (Baltimore) 2016;95:e3592.
18. Singhai R, Patil V, Patil A. Immunohistochemical (IHC) HER-2/neu and fluorescent-in situ hybridization (FISH) gene amplification of breast cancer in Indian women. Asian Pac J Cancer Prev 2011;12:179-83.
19. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987;235:177-82.
20. Goud KI, Dayakar S, Vijayalaxmi K, Babu SJ, Reddy PV. Evaluation of HER-2/neu status in breast cancer specimens using immunohistochemistry (IHC) & fluorescence in-situ hybridization (FISH) assay. Indian J Med Res 2012;135:312-7.
21. Pauletti G, Dandekar S, Rong H, Ramos L, Peng H, Seshadri R, et al. Assessment of methods for tissue-based detection of the HER-2/neu alteration in human breast cancer: a direct comparison of fluorescence in situ hybridization and immunohistochemistry. J Clin Oncol 2000;18:3651-64.
22. Pehlivanoglu B, Serin G, Yeniay L, Zekioglu O, Gokmen E, Ozdemir N. Comparison of HER2 status determination methods in HER2 (2+) patients: manual fluorescent in situ hybridization (FISH) vs. dual silver enhanced in situ hybridization (SISH). Ann Diagn Pathol 2017;31:36-40.
23. Mass RD, Press MF, Anderson S, Cobleigh MA, Vogel CL, Dybdal N, et al. Evaluation of clinical outcomes according to HER2 detection by fluorescence in situ hybridization in women with metastatic breast cancer treated with trastuzumab. Clin Breast Cancer 2005;6:240-6.
24. Toi M, Sperinde J, Huang W, Saji S, Winslow J, Jin X, et al. Differential survival following trastuzumab treatment based on quantitative HER2 expression and HER2 homodimers in a clinic-based cohort of patients with metastatic breast cancer. BMC Cancer 2010;10:56.
25. Lipton A, Köstler WJ, Leitzel K, Ali SM, Sperinde J, Weidler J, et al. Quantitative HER2 protein levels predict outcome in fluorescence in situ hybridization-positive patients with metastatic breast cancer treated with trastuzumab. Cancer 2010;116:5168-78.
26. Xu QQ, Pan B, Wang CJ, Zhou YD, Mao F, Lin Y, et al. HER2 amplification level is not a prognostic factor for HER2-positive breast cancer with trastuzumab-based adjuvant treatment: a systematic review and meta-analysis. Oncotarget 2016;7:63571-82.
27. Chibon F, de Mascarel, Sierankowski G, Brouste V, Bonnefoi H, Debled M, et al. Prediction of HER2 gene status in Her2 2+ invasive breast cancer: a study of 108 cases comparing ASCO/CAP and FDA recommendations. Mod Pathol 2009;22:403-9.
28. Ohnstad HO, Borgen E, Falk RS, Lien TG, Aaserud M, Sveli MA, et al. Prognostic value of PAM50 and risk of recurrence score in patients with early-stage breast cancer with long-term follow-up. Breast Cancer Res 2017;19:120.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||