INTRODUCTION
Granular cell tumor (GCT) is a rare neoplasm that originates from Schwann cells in the nervous system. Although GCT can arise at any site in the body, it is usually found in the oral cavity, skin, or the digestive or respiratory tracts [1,2]. GCT predominantly occurs in women and may arise in the breast, and closely resemble carcinoma clinically, radiologically, and ultrasonographically [3,4]. The mammographic findings of GCT are those of an ill-defined heterogeneous dense mass [3], whereas ultrasonography reveals the presence of an irregularly shaped mass-like malignancy [4]. Some authors have previously reported GCT of the breast, but to the best of our knowledge, there is no report of a newly developed GCT in the contralateral breast after wide excision of a previous GCT lesion. Here, we describe a case of newly arising GCT in the contralateral breast 1 year after excision of a large breast GCT.
CASE REPORT
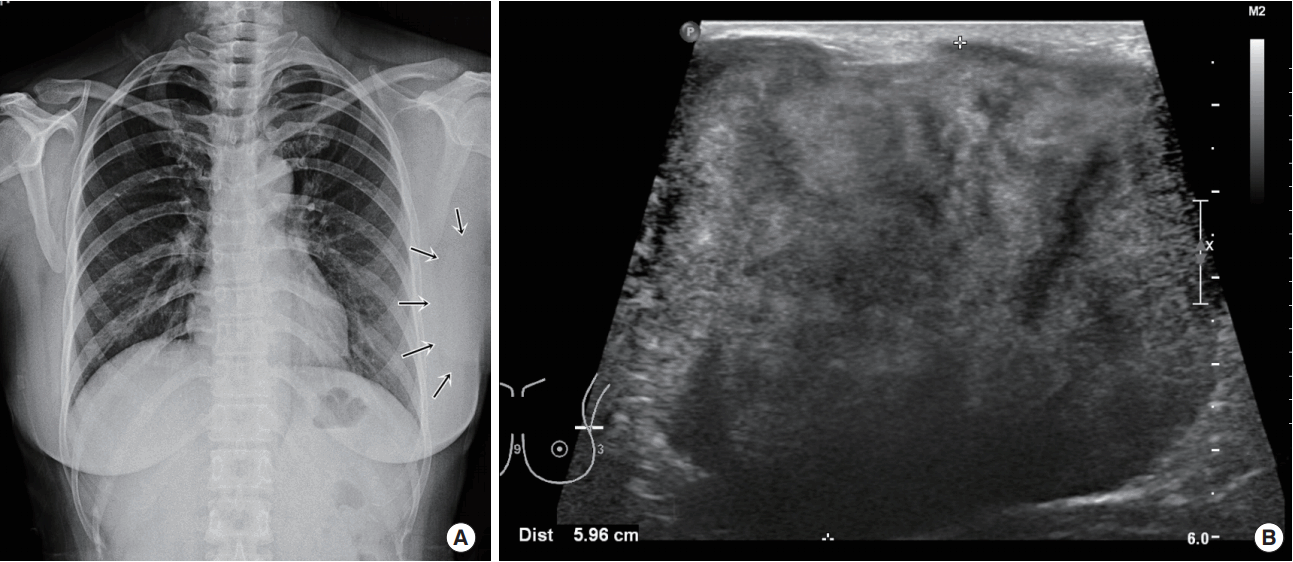
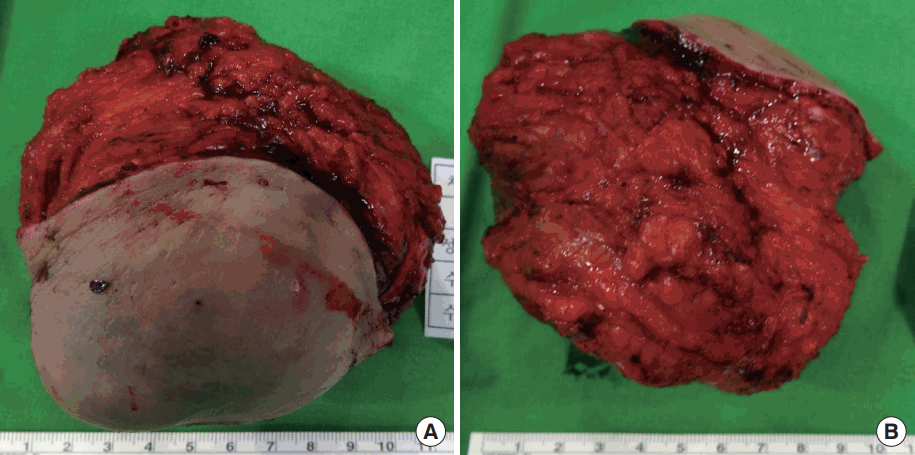
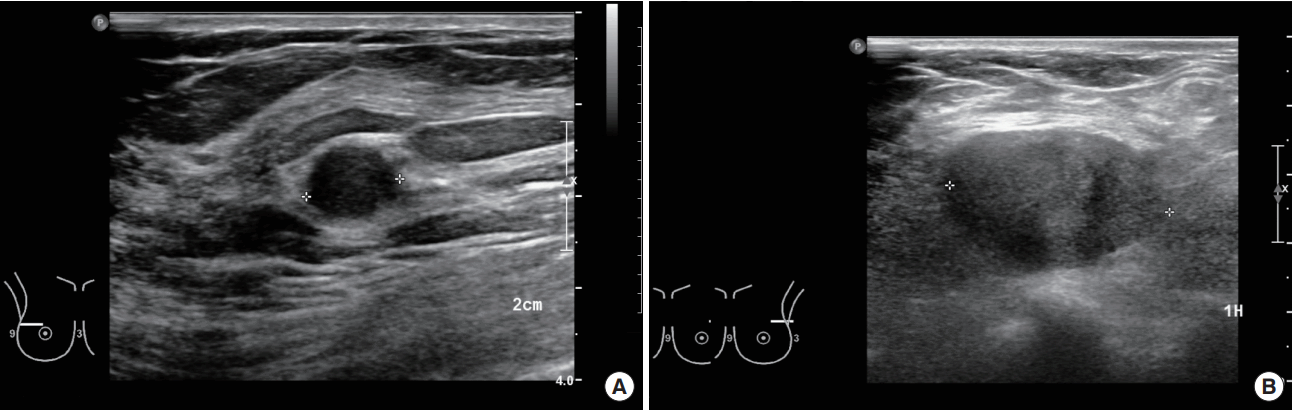
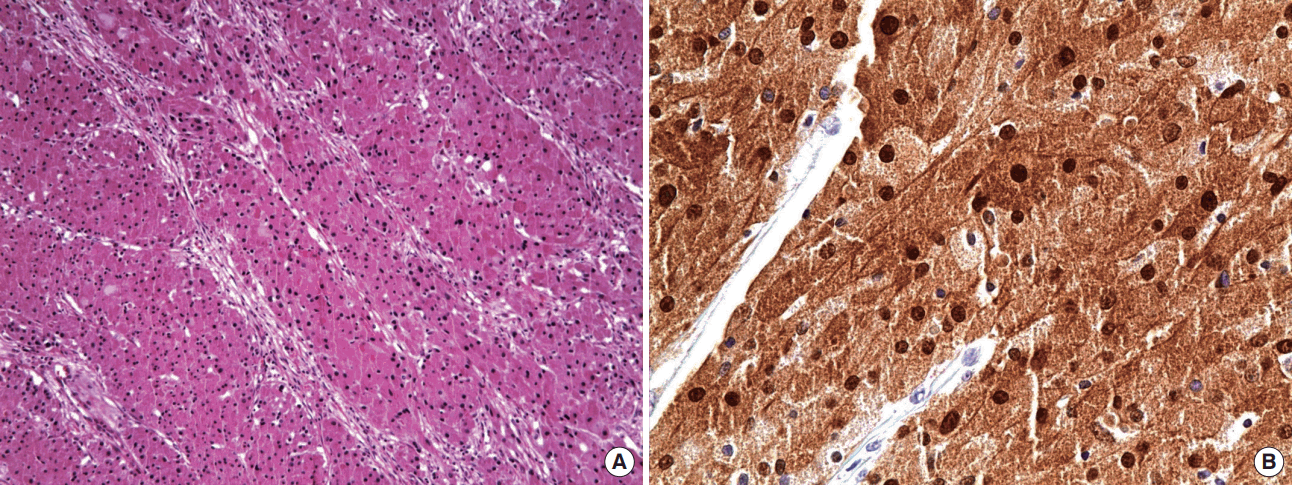
A 46-year-old woman presented with a painful, palpable large left breast mass that extended to the ipsilateral axilla. She had incidentally found a small lump in the upper lateral quadrant of her left breast 10 years prior to the presentation, but she did not seek medical advice because she did not experience any pain and the mass grew slowly. However, 1 month before this initial presentation, the patient felt blunt pain on the left breast lesion. On physical examination, the mass was found to be firm, well-defined, fixed, and about 10 cm in size with bulging of the overlying skin. Chest posterior-anterior radiography showed a large mass shadow, and ultrasonography revealed the presence of a large homogeneous solid mass in the left axillary area (Figure 1), but no mass or abnormal findings in the right breast. The patient had no history of surgery or use of any medication. Wide excision (Figure 2) was performed under general anesthesia, and pathologic findings revealed the mass to be a benign GCT (Figure 3), with tumor-free resection margins. Immunohistochemistry of the resected specimen was positive for Ki-67 (less than 10% of tumor cells), S-100, and CD-68, but negative for cytokeratin and vimentin (not illustrated). No postoperative complication was observed, and the patient reported that he did not have any discomfort. Six months after the surgery, we found 1-cm masses in both breasts and the patient was closely observed. A year after the initial surgery, the left breast mass grew to 3 cm in the previous operated site, and the contralateral well-defined mass, which was approximately 1.2 cm in size, was in the upper lateral quadrant of the right breast (Figure 4). Excision biopsy of both breast masses was performed and the histopathologic results were similar to those observed after the first surgery. Pathologic results indicated benign GCTs with numerous aggregates of cells containing small round bland nuclei and abundant granular cytoplasm. Immunohistochemistry was strongly positive for S-100 (Figure 5).
DISCUSSION
GCT of the breast is relatively uncommon and has been reported to account for approximately 9% of all GCTs [2]. First described in 1926 by Abrikossoff, GCT was initially presumed to be of myogenic origin, but recently, its strong immunoreactivity to S-100 protein revealed that it originates from Schwann cells [5,6]. In most cases, even in patients with multiple and atypical GCTs, the tumor remains benign in nature, but in malignant cases, GCT is associated with a local recurrence rate of 32%, a metastasis rate of 50%, and mortality rate of 39% [6]. However, there are no specific findings to distinguish GCT from other types of breast lesions. Clinically, rapid growth, large tumor size (>4 cm), invasion into adjacent tissues, local recurrence, and an advanced age have been reported to be poor prognostic factors [7]. Nonspecific physical examination, mammographic, and ultrasound findings might not contribute to the diagnosis of GCT. In fact, only histopathologic confirmation after surgical excision provides a diagnosis of breast GCT. In the described case, the patient presented with a large left breast mass (>10 cm), and we were unable to determine whether it was malignant or benign based on ultrasonographic findings. For prompt diagnosis and treatment, we performed a wide excision rather than needle biopsy, and explained to the patient of the possibility of second operation based on the histopathologic results.
Fanburg-Smith et al. [6] suggest six histopathologic criteria for detecting malignant GCT. These features include high nuclear-to-cytoplasmic ratio, nuclear pleomorphism, necrosis, spindle-shaped tumor cells, vesicular nuclei with prominent nucleoli, and increased mitotic activity (>2 mitoses per 10 high-power fields at 200× magnification). Based on the above criteria, GCTs are classified as benign (satisfy 0 criteria or focal pleomorphism), atypical (satisfy 1–2 criteria), or malignant (satisfy more than 3 criteria) [6]. Furthermore, high Ki-67 expression was revealed in a majority of malignant cases [8]. In this case, only focal necrosis was consistent with malignant GCT that we classified as benign. Despite histopathologic confirmation of the benign nature of the GCT, and the clear surgical margins achieved, the left side mass recurred and a new right side mass developed a year after the initial surgery. The recurrence rate of benign GCT is 2% to 8 % with a clear resection margin, and this increases to 20% when resection margins are positive for tumor infiltration [6]. Our patient had some poor prognostic factors, such as large tumor size, local recurrence, and a new mass arising in the right breast. The treatment of GCT is clear and straightforward. Even for malignant GCT, only surgical resection is recommended. Chemotherapy and/or radiotherapy do not improve clinical course [9]. We also performed wide excision of GCTs in both breasts.
GCT of the breast is a rare neoplasm that rarely results in de novo development in the contralateral breast. It is difficult to differentiate benign and malignant lesions based on physical examination or imaging findings alone, and thus, initial diagnosis should further be confirmed by biopsy or complete surgical resection.