AbstractPurposeThe molecular subtype of breast cancer is an important predictive factor. Therefore, we investigated the effects of concurrent or serial radiotherapy and systemic therapy on metastatic brain lesions according to the molecular subtype of breast cancer.
MethodsThe present retrospective study examined data from 66 patients with breast cancer and metastatic brain lesions, who were treated using radiotherapy between January 1990 and July 2014. Patients were classified into the following three subtypes based on their hormone receptor (HR) and human epidermal growth factor receptor 2 (HER2) status: HR+/HER2− (luminal A, 13 patients), HR+/HER2+ (luminal B, 21 patients), HR−/HER2+ (HER2, 22 patients), or HR−/HER2− (triple negative, 10 patients). The brain lesions and their responses to treatment were evaluated using brain computed tomography or magnetic resonance imaging. Progression of brain disease was defined by a ≥20% increase in the sum of the lesion’s diameters or the development of a new brain lesion. Progression-free survival was calculated from the initiation of radiotherapy to the first instance of brain disease progression or last follow-up.
ResultsPatients in the HER2 group who had received concurrent radiotherapy and systemic therapy (mainly HER2-targeted therapy) exhibited significantly better progression-free survival than did patients who had received radiotherapy followed by systemic therapy (p=0.037). However, concurrent radiotherapy and systemic therapy did not significantly improve progression-free survival in the luminal A (p=0.527), luminal B (p=0.462), or triple negative (p=0.558) groups.
INTRODUCTIONRadiotherapy is an important treatment option for patients with breast cancer. It is essential to achieve the same level of local control as total mastectomy after breast-conserving surgery [1]. Adjuvant radiotherapy could also improve the overall survival of patients with breast cancer [2,3]. In the metastatic setting, administering radiotherapy is also an effective strategy. Especially for brain metastasis of breast cancer, radiotherapy is a standard treatment. However, there are no validated clinical factors for predicting the outcomes of radiotherapy.
A Danish Breast Cancer Cooperative Group subgroup analysis of overall survival according to molecular subtype revealed that the hormone receptor (HR) and human epidermal growth factor receptor 2 (HER2) statuses might predict the outcomes of radiotherapy [4]. Furthermore, in our previous retrospective nationwide analysis, postoperative radiotherapy was not associated with a survival benefit among patients with HER2+ breast cancer (p=0.887) whereas patients with HER2− breast cancer experienced better overall survival after radiotherapy treatment (p=0.037) [5]. Interestingly, preclinical studies have suggested that HER2 overexpression is related to radiotherapy resistance, as transfection of breast cancer cells with HER2 gene caused radiotherapy resistance [6,7]. Moreover, several selective inhibitors that target downstream of HER2 signaling can induce radiosensitivity in breast cancer cells overexpressing HER2 [8]. Therefore, we hypothesized that the molecular subtype, particularly the HER2 status, might be associated with response to radiotherapy, and that concurrent radiotherapy and systemic HER2-targeted therapy might be more effective than radiotherapy followed by systemic therapy in patients with HER2-positive breast cancer.
As mentioned above, radiotherapy is the standard treatment for metastatic brain lesions [9-12]. The local treatment of brain metastases could be achieved via whole brain radiotherapy (WBRT), stereotactic radiosurgery (SRS), and/or surgery although the prognosis of brain metastasis is poor [12]. Systemic therapy including HER2-targeted therapy is recommended for patients with brain metastases to control extracranial disease after radiotherapy [12]. While adjuvant radiotherapy was performed without residual tumor after effective surgery, the dimensions of the metastatic brain lesion could be measured and the direct effect of radiotherapy with or without concurrent therapy could be evaluated. Thus, we chose metastatic brain lesions as a human in vivo model to investigate the effect of radiotherapy with or without concurrent systemic therapy according to the subtype of breast cancer. This model could be useful for evaluating the tumor response to radiotherapy with or without concurrent systemic therapy. Therefore, in the present study, we retrospectively evaluated and compared the effects of concurrent radiotherapy and systemic therapy on metastatic brain lesions to those of radiotherapy followed by systemic therapy depending on the molecular subtype of the primary breast cancer.
METHODSWe retrospectively analyzed the data from patients with breast cancer and metastatic brain lesions who underwent either WBRT or SRS between January 1990 and July 2014. The WBRT was prescribed at a total dose of 30 Gy delivered in 10 fractions of 3 Gy per day. WBRT was delivered from a linear accelerator using 4 or 6 MV lateral opposed photon fields. The SRS was prescribed at a total dose of 15–24 Gy administered in a single fraction according to the size and location of the metastatic tumor. SRS was delivered using CyberKnife (Accuray Co., Sunnyvale, USA).
Patients who completed the planned course of radiotherapy were included. Patients who did not undergo brain computed tomography (CT) or magnetic resonance imaging (MRI) during the follow-up period as well as patients with incomplete data of estrogen receptor (ER), progesterone receptor (PR), and HER2 statuses, were excluded. The present study was approved by the Institutional Review Board of the Korea Cancer Center Hospital (K-1410-002-084).
Patients were classified into the following three subtypes based on the HR and HER2 statuses of their primary breast cancers: HR+/HER2− (luminal A group), HR+/HER2+ (luminal B group), HR−/HER2+ (HER2 group), or HR−/HER2− (triple negative group). HR positivity was defined by ER positivity or PR positivity using immunohistochemistry, and HER2 positivity was defined by an immunohistochemistry score of 3+ or positive results obtained from fluorescence in situ hybridization or silver in situ hybridization. The dimensions of the metastatic brain lesions were measured using contrast-enhanced brain CT or MRI, and the same modality was used to evaluate the treatment response in each patient. The longest tumor diameter was measured on the axial slice of the brain CT or MRI images. Response to radiotherapy was evaluated using the change in size of the target lesion after radiotherapy. If the patient had multiple metastatic brain lesions, the largest five lesions were selected as the target lesions. The target lesions were added together and evaluated, and brain disease progression was defined by a ≥20% increase in the sum of diameters of the target lesions or the development of a new brain lesion. Progression-free survival was defined as the time from the initiation of radiotherapy to the first instance of brain disease progression, last follow-up, or death. Concurrent therapy was defined as the administration of radiotherapy at the same time as systemic therapy, and serial therapy was defined as the administration of radiotherapy followed by systemic therapy. The systemic therapies that the patients received included cytotoxic chemotherapy, HER2-targeted therapy, and endocrine therapy.
Survival analysis was performed using the Kaplan–Meier method and the results were compared using the log-rank test. The Pearson chi-square test was used to assess differences in the clinicopathological factors between the groups. All results with p-values <0.05 were considered statistically significant. Data analysis was performed using SPSS version 22 (SPSS Inc., Armonk, USA).
RESULTSIn total, 66 patients were eligible for analysis. The median age was 46.9 (range, 21.2–72.6) years, the median time from breast cancer diagnosis to brain metastasis was 9.0 (range, 0–17.9) years, and the median follow-up period was 9.7 (range, 1.6–65.3) months. The median disease-free survival of all patients was 29.4 (range, 22.3–36.6) months. The median overall survival from the diagnosis to death of all patients was 78.3 (range, 31.8–124.9) months.
Thirty-two patients had received concurrent therapy, and 34 patients had received serial therapy. Approximately 31.8% of the patients in the HER2 group were <50 years old. There was no significant difference in the nodal status or distant metastasis at the time of diagnosis. In the luminal A group, the proportion of patients treated with SRS was higher than those of the other groups (p=0.004). Most of the patients had received adjuvant chemotherapy (Table 1). Other metastatic sites that coexisted with the brain lesions when the brain metastasis was diagnosed are described in Table 2.
There were no statistically significant differences in the brain disease progression-free survivals between the groups (p=0.757, Figure 1). However, there were significantly different tumor responses when we compared the patients who received concurrent and serial therapies depending on the tumor subtypes (Figure 2). In the HER2 group, patients who had received concurrent therapy exhibited a significantly better brain disease progression-free survival compared to patients who had received serial therapy (p=0.037, Figure 2C). Among the patients who had received concurrent therapy in the HER2 group, six patients (6/8, 75.0%) were treated using systemic HER2-targeted therapy (trastuzumab or lapatinib). None of the patients had previously been treated with trastuzumab emtansine or pertuzumab. Five patients (5/8, 62.5%) received the same post-diagnosis systemic therapy that was being used before their diagnosis of brain metastasis, and four of these patients had underwent HER2-targeted therapy (Table 1).
DISCUSSIONIn the HER2 group, patients who had received concurrent radiotherapy and systemic therapy exhibited significantly better progression-free survival compared to patients who had received serial radiotherapy followed by systemic therapy. However, the benefits of concurrent therapy were not observed in the other subtype groups. Furthermore, to the best of our knowledge, this is the first report to describe the benefit of concurrent radiotherapy and systemic therapy over radiotherapy followed by systemic therapy in the treatment of metastatic brain lesions among patients with HER2+ disease.
Radiotherapy may induce changes in the blood-brain barrier (BBB) permeability, which may explain the benefit of concurrent radiotherapy and systemic therapy on progression-free survival. In an animal model of breast cancer metastasis, the residual brain-tumor barrier generally limited chemotherapy (doxorubicin and paclitaxel) distribution to subtherapeutic levels in the brain, irrespective of the fact that most brain metastases impaired the brain-tumor barrier [13]. These findings clearly highlight the requirement for new drugs that can pass the BBB (e.g., lapatinib) or new treatment modalities that can increase the BBB permeability, thus allowing effective delivery of therapeutic agents to the brain [14]. In this context, a total radiotherapy dose of 20–30 Gy delivered in 2 Gy fractions can increase the BBB permeability, which might allow the therapeutic agent to pass the BBB and exert its therapeutic effect [15]. Furthermore, Ott et al. [16] had measured the BBB permeability in patients undergoing radiotherapy and chemotherapy for primary cerebral lymphoma, using time-sequence positron emission tomography with a radioactive tracer (68Ga-EDTA). These investigators reported significant permeability changes in the tumor area after radiotherapy, but not around the normal brain, although the permeability near the tumor returned to the level of the normal brain within 5 weeks. Therefore, it appears possible that the better tumor response to concurrent radiotherapy and systemic therapy might be related to radiotherapy-induced changes in the BBB permeability. It is unclear why this benefit was limited to the HER2 group; however, we hypothesize that it could be related to the resistance of HER2+ cells to radiotherapy and the ability of HER2-targeted therapy to sensitize these cells to radiotherapy.
Few clinical studies were conducted to investigate the resistance of breast cancer to radiotherapy according to HER2 status. After a median follow-up duration of 17 years, the Danish Breast Cancer Cooperative Group reported a significantly improved overall survival after post-mastectomy radiotherapy among patients with HR+ and HER2− breast cancers. However, there was no significant improvement in the overall survival after post-mastectomy radiotherapy among patients with HR− and HER2+ breast cancers [4]. Furthermore, our nationwide analysis of 11,552 patients with T1N0M0 breast cancers revealed a significant improvement in the overall survival after radiotherapy in the HER2− group (p=0.037) while it did not confer a significant survival benefit in the HER2+ group (p=0.887) [5]. Moreover, these findings obtained in the adjuvant setting are supported by data from several preclinical studies [6,8,17,18]. For example, radiotherapy resistance was induced when the MCF7 cells, which exhibit standard HER2 expression, were transfected with the HER2 gene for overexpression [6].
Interestingly, the radiotherapy resistance of MCF7 cells that overexpressed HER2 was reduced when the cells were treated using trastuzumab [6,18]. Furthermore, HER2-positive breast cancer cells that were treated with selective inhibitors of the PI3K-AKT-mTOR pathway exhibited significantly attenuated expression of p-AKT and p-70S6K, and became sensitized to radiotherapy [8,18]. Therefore, our findings in the present study agree with the findings from these preclinical experiments, as most of the systemic therapies included HER2-targeted agents, which would suggest that the benefit of concurrent therapy might have been a consequence of radiotherapy sensitivity conferred by HER2-targeted therapy. However, this possibility raises the question of whether HER2-targeted therapy can pass the BBB. For example, lapatinib is a small molecule that can pass through the BBB and diffuse into the brain lesions [14]. Although trastuzumab is a large molecule that normally cannot pass the BBB, it was used in the treatment of 4 patients in the HER2 group that received concurrent therapy. Another study had reported that 64Cu-DOTA-trastuzumab could be used with positron emission tomography to identify primary or metastatic HER2+ breast cancer [19,20]. Thus, it would appear that trastuzumab could at least pass the BBB; however, there are no data confirming whether trastuzumab reaches therapeutic concentrations in the brain. Furthermore, it is possible that HER2-targeted therapies might pass though the BBB and exert direct therapeutic effects on the lesion instead of indirect effects to increase the radiosensitivity of the lesion. However, all patients who had received concurrent radiotherapy and trastuzumab-containing therapy experienced initial brain metastases during trastuzumab treatment, which would indicate that they had trastuzumab-refractory disease. Therefore, it appears unlikely that the benefit of concurrent radiotherapy and trastuzumab-containing therapy was related to a direct effect of trastuzumab on the metastatic brain lesions, and we speculate that this effect might be related to a radiotherapy-induced increase in BBB permeability, which would allow HER2-targeted therapy to cross the BBB and induce radiosensitivity in the metastatic brain lesions.
The limitations of the present study were its retrospective design and small sample size, and, the proportion of elderly patients of HER2 group was higher than in other patient groups. Therefore, larger prospective studies should be conducted to confirm our findings. However, our results concerning the size of the target lesions are likely accurate, as we performed consistent follow-ups using CT or MRI, which would not typically be used in clinical studies conducted in the adjuvant setting that typically examine disease-free or overall survival.
In conclusion, concurrent radiotherapy and systemic therapy (mainly HER2-targeted therapy) significantly improved the progression-free survival among patients with HER2+ brain disease. We speculate that this might be related to the resistance of HER2+ breast cancer to radiotherapy being overcome by concurrent radiotherapy and HER2-targeted therapy, although larger prospective studies are required to confirm this hypothesis.
ACKNOWLEDGMENTSThe present study was supported by a grant from the Korea Institute of Radiological and Medical Sciences (KIRAMS), which is funded by the Ministry of Science, ICT and Future Planning, Republic of Korea (1711031800/50451-2016).
Figure 2.The effect of concurrent therapy on progression-free survival in the (A) luminal A group, (B) luminal B group, (C) HER2-positive group, and (D) triple negative group. 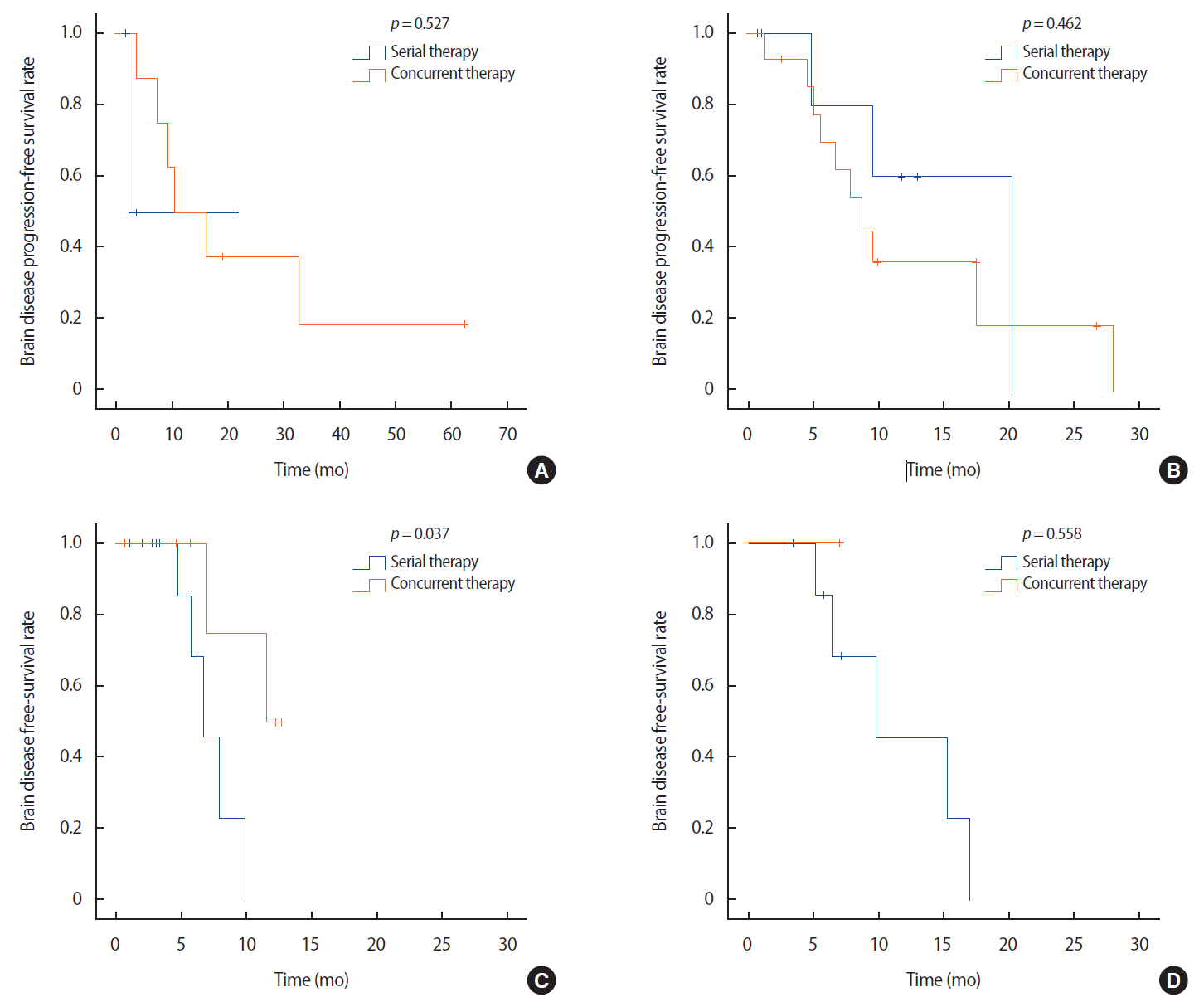
Table 1.Clinicopathologic characteristics of patients Table 2.Site of distant metastasis with brain metastasis REFERENCES1. Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002;347:1233-4.
2. Overgaard M, Jensen MB, Overgaard J, Hansen PS, Rose C, Andersson M, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet 1999;353:1641-8.
3. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Darby S, McGale P, Correa C, Taylor C, Arriagada R, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011;378:1707-16.
4. Kyndi M, Sørensen FB, Knudsen H, Overgaard M, Nielsen HM, Overgaard J, et al. Estrogen receptor, progesterone receptor, HER-2, and response to postmastectomy radiotherapy in high-risk breast cancer: the Danish Breast Cancer Cooperative Group. J Clin Oncol 2008;26:1419-26.
5. Kim HA, Kim EK, Kim MS, Yu JH, Lee MR, Lee HK, et al. Association of human epidermal growth factor receptor 2 with radiotherapy resistance in patients with T1N0M0 breast cancer. J Breast Cancer 2013;16:266-73.
6. Pietras RJ, Poen JC, Gallardo D, Wongvipat PN, Lee HJ, Slamon DJ. Monoclonal antibody to HER-2/neureceptor modulates repair of radiation-induced DNA damage and enhances radiosensitivity of human breast cancer cells overexpressing this oncogene. Cancer Res 1999;59:1347-55.
7. Pirollo KF, Tong YA, Villegas Z, Chen Y, Chang EH. Oncogene-transformed NIH 3T3 cells display radiation resistance levels indicative of a signal transduction pathway leading to the radiation-resistant phenotype. Radiat Res 1993;135:234-43.
8. No M, Choi EJ, Kim IA. Targeting HER2 signaling pathway for radiosensitization: alternative strategy for therapeutic resistance. Cancer Biol Ther 2009;8:2351-61.
9. Alexander E 3rd, Moriarty TM, Davis RB, Wen PY, Fine HA, Black PM, et al. Stereotactic radiosurgery for the definitive, noninvasive treatment of brain metastases. J Natl Cancer Inst 1995;87:34-40.
10. Mahmoud-Ahmed AS, Suh JH, Lee SY, Crownover RL, Barnett GH. Results of whole brain radiotherapy in patients with brain metastases from breast cancer: a retrospective study. Int J Radiat Oncol Biol Phys 2002;54:810-7.
11. Melisko ME, Moore DH, Sneed PK, De Franco J, Rugo HS. Brain metastases in breast cancer: clinical and pathologic characteristics associated with improvements in survival. J Neurooncol 2008;88:359-65.
12. Niikura N, Saji S, Tokuda Y, Iwata H. Brain metastases in breast cancer. Jpn J Clin Oncol 2014;44:1133-40.
13. Lockman PR, Mittapalli RK, Taskar KS, Rudraraju V, Gril B, Bohn KA, et al. Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin Cancer Res 2010;16:5664-78.
14. Gril B, Palmieri D, Bronder JL, Herring JM, Vega-Valle E, Feigenbaum L, et al. Effect of lapatinib on the outgrowth of metastatic breast cancer cells to the brain. J Natl Cancer Inst 2008;100:1092-103.
15. van Vulpen M, Kal HB, Taphoorn MJ, El-Sharouni SY. Changes in blood-brain barrier permeability induced by radiotherapy: implications for timing of chemotherapy? (Review). Oncol Rep 2002;9:683-8.
16. Ott RJ, Brada M, Flower MA, Babich JW, Cherry SR, Deehan BJ. Measurements of blood-brain barrier permeability in patients undergoing radiotherapy and chemotherapy for primary cerebral lymphoma. Eur J Cancer 1991;27:1356-61.
17. Sambade MJ, Kimple RJ, Camp JT, Peters E, Livasy CA, Sartor CI, et al. Lapatinib in combination with radiation diminishes tumor regrowth in HER2+ and basal-like/EGFR+ breast tumor xenografts. Int J Radiat Oncol Biol Phys 2010;77:575-81.
18. Liang K, Lu Y, Jin W, Ang KK, Milas L, Fan Z. Sensitization of breast cancer cells to radiation by trastuzumab. Mol Cancer Ther 2003;2:1113-20.
|
|
||||||||||||||||||||||||||||||||||