AbstractPurpose:We aimed to evaluate the incidence of multifocal and bilateral lobular carcinoma in situ (LCIS) of the breast in Korean women. Additionally, we explored the characteristics of current breast imaging studies and evaluated their roles in detecting multifocal and bilateral lesions in LCIS patients.
Methods:Between January 2000 and December 2013, we identified 48 patients with pure LCIS who underwent curative surgery at our institution. The pathological findings and the results of various imaging studies were reviewed.
Results:All patients underwent mammography and ultrasonography prior to surgery, and 35 patients (72.9%) also received breast magnetic resonance imaging (MRI). The most common radiologic features of LCIS were the presence of microcalcifications (66.7%) and irregularly shaped masses (64.6%) on mammography and ultrasonography, respectively. MRI often showed the presence of irregularly enhanced mass-like lesions (91.4%). Additional suspicious lesions in the ipsilateral breast were identified in 19 (39.6%) and 16 (45.7%) patients on ultrasonography and MRI, respectively. Among them, nine (47.4%) were malignant lesions. In the contralateral breast, there were 14 (29.2%) and 11 (31.4%) lesions requiring biopsy on ultrasonography and MRI, respectively, and eight of these were found to be malignant lesions. All suspicious lesions detected by MRI were also seen on ultrasonography. In total, 20.8% (10/48) of the patients had ipsilateral LCIS, and 16.7% (8/48) had contralateral malignancies.
INTRODUCTIONLobular carcinoma in situ (LCIS) is an uncommon noninvasive lesion first documented as an “atypical proliferation of acinar cells” by Ewing [1] in 1919. In 1941, Foote and Stewart [2] first designated the lesion as LCIS and described it as a precancerous lesion of the proliferative mammary epithelium. As previously suggested in a study by Foote and Stewart [2], the presence of LCIS can be a risk stratifier and may represent a precancerous stage in the multistep process of carcinogenesis. Indeed, the following studies have shown that unlike ductal carcinoma in situ (DCIS), LCIS is more likely to be a risk factor rather than a true early cancerous lesion [3-6]. LCIS is often discovered incidentally from biopsies performed on the breast for other reasons. Accordingly, the incidence of LCIS in the general population is difficult to accurately determine. Nonetheless, the incidence of LCIS in otherwise benign breast biopsies is reported to be 0.02% to 3.8% [3,7,8]. The Surveillance, Epidemiology, and End Results data from 1978 to 1998 showed that the highest incidence rate of LCIS was 3.19/100,000 person-years, which is substantially lower than that of DCIS (12.73/100,000 person-years) during the same period [9]. This relatively low incidence of LCIS is a main reason for the prevailing controversy in the diagnosis and treatment of this disease.
Early studies have reported high rates of multifocal and bilateral lesions in patients with LCIS. In a case series of patients who underwent contralateral biopsy procedures during curative surgery for ipsilateral LCIS, more than 50% of the patients had multifocal or multicentric lesions, and one-third had bilateral lesions [10-12]. However, it is widely accepted that LCIS usually does not present characteristic abnormal findings in conventional imaging studies [13,14], impeding the detection of these additional lesions. Pope et al. [13] reviewed the imaging findings of 26 patients with LCIS and reported that most LCIS cases do not show distinctive mammography (MMG) features, with the exception of a small proportion of patients with MMG calcification or asymmetry. Sonnenfeld et al. [14] reviewed 41 consecutive cases of LCIS and also concluded that most of the microcalcifications that resulted in the diagnosis of LCIS were due to the benign pathological process occurring near the LCIS.
The accuracy and sensitivity of breast imaging have improved dramatically during the last few decades. Therefore, the ability of modern breast imaging studies, including MMG, breast ultrasonography (USG), and magnetic resonance imaging (MRI) to detect multifocal or bilateral LCIS should be re-evaluated. The purpose of this study was to evaluate the incidence of multifocal and bilateral LCIS of the breast in Korean women. Additionally, we explored the characteristics and roles of the current breast imaging studies in detecting multifocal and bilateral lesions in LCIS patients.
METHODSThis retrospective study was approved by the Institutional Review Board of Seoul National University Hospital (IRB number: 1410-028-616) and conducted in accordance with the Helsinki Declaration. The requirement for informed consent was waived.
Patient selectionWe searched for patients with no breast abnormalities other than LCIS in the initially detected lesion in the database of Seoul National University Hospital Breast Care Center (SNUHBCC). The SNUHBCC database is a prospectively maintained, web-based system that includes information on all patients who have undergone operations for breast diseases at Seoul National University Hospital since 1982 [15]. Of more than 12,000 patients registered in the Seoul National University Hospital Breast Cancer Center database between January 2000 and December 2013, we retrieved the data of 357 patients who were diagnosed with LCIS. Among the 357 patients, most had LCIS as an incidental pathological finding in addition to their invasive breast carcinomas. After excluding these patients, we were able to identify 48 pure LCIS patients. The clinical and pathological data, as well as the radiological test results, were retrieved from the electronic medical records.
Breast imagingAll patients underwent preoperative MMG (Mammomat 3000, Siemens Medical Solutions, Solna, Sweden, or Lorad M3 mammography unit, Hologic, Boston, USA) and breast USG (HDI 5000 scanner or iU22 scanner with a 7- to 15-MHz linear probe; Philips Ultrasound, Bothell, USA). Preoperative MRI was performed in 35 patients using a 1.5-T system (Signa®; General Electric Medical Systems, Milwaukee, USA) or a commercially available 3.0-T system (Magnetom Verio; Siemens Medical Solutions, Erlangen, Germany). Fat-suppressed T2-weighted fast spin-echo images (repetition time [TR]/echo delay time [TE], ranging from 5,500 to 7,150/82 ms; matrix, 256×160; field of view, 200× 200 mm2; slice thickness, 1.5 mm) and dynamic contrast-enhanced images, including one precontrast and five postcontrast sagittal images using a fat-suppressed T1-weighted 3D fast spoiled gradient echo sequence (TR/TE, 6.5/2.5 ms; matrix, 256×160; flip angle, 10°; field of view, 200× 200 mm; slice thickness, 1.5 mm), were obtained. The lesion characteristics were documented using the American College of Radiology Breast Imaging Reporting and Data System (BI-RADS) lexicons [16].
RESULTSClinicopathological characteristics of patientsAmong patients who underwent breast surgery between January 2000 and December 2013, we identified 48 patients who were diagnosed with pure LCIS. The demographic and clinicopathological characteristics of the patients included in the study are listed in Table 1. Thirty-six patients were asymptomatic, and their imaging abnormalities were detected through MMG screening and/or USG. Twenty-five of the LCIS lesions were located in the left breast; the other 20 were located in the right breast. The incidence of estrogen receptor and progesterone receptor positivity was 90.0% (18/20) and 83.3% (15/18), respectively.
Imaging characteristics of initially biopsied LCIS lesionsWe evaluated the imaging findings of the LCIS lesions by reviewing MMG, USG, and MRI images of the studied patients. Mammography and USG were performed in all patients, and MRI images were available for 35 patients (72.9%). The imaging characteristics on MMG, USG, and MRI of the initially biopsied lesions are listed in Table 2. The most common MMG abnormality in patients with LCIS was the presence of microcalcification (n =32, 66.7%). All eight patients without MMG abnormalities had positive USG findings, and six of these patients had also undergone an MRI that showed abnormalities at the same location. Similarly, abnormalities on MMG and MRI were noted in the two patients with no abnormality on USG. The most common USG feature in the remaining 46 patients was an irregularly shaped mass (n =31, 64.6%). In 32 patients, the contrast-enhanced breast MRI showed irregularly enhanced mass-like lesions, while three patients showed non-mass-like enhancement. Interestingly, the radiologic appearance of many LCIS lesions suggested a low suspicion for malignancy. Thirty-five patients (72.9%) showed a BIRADS classification C4a on USG, and 21 patients (60.0%) showed a BI-RADS classification C4a on MRI.
Multifocal/multicentric and bilateral lesionsIn the 48 LCIS patients, additional imaging abnormalities in the ipsilateral breast requiring biopsy were detected in 19 (39.6%) and 16 (45.7%) patients on USG and MRI, respectively. All additional lesions seen on MRI were also visible on USG and were subjected to biopsies, although one was confirmed on a second USG after it was detected on MRI. Among them, 10 lesions (52.6%) were LCIS, and the remaining nine were benign lesions. Contralateral imaging abnormalities requiring biopsy were detected in 14 (29.2%) and 11 patients (31.4%) on USG and MRI, respectively. Among them, only one showed pure LCIS in the contralateral breast. Seven lesions were diagnosed as malignant tumors such as invasive ductal carcinoma, invasive lobular carcinoma, and DCIS. Among the seven malignant lesions, two had surrounding LCIS lesions. In total, 20.8% (10/48) of the patients had ipsilateral LCIS, and 16.7% (8/48) had contralateral malignancies (Figure 1). Representative examples of breast imaging studies in a patient with additional multicentric and bilateral lesions in patients diagnosed with LCIS are shown in Figure 2.
DISCUSSIONIn this study, we reviewed the clinicopathological characteristics and imaging results for 48 patients who were diagnosed with pure LCIS, and we evaluated the incidence of multifocal/multicentric or bilateral lesions detected by multiple breast imaging modalities. The most commonly observed finding on MMG and USG was microcalcification in 66.7% of patients and an irregularly shaped mass in 64.6% of patients, respectively. Among 19 additional imaging abnormalities in the ipsilateral breast, 10 multifocal/multicentric lesions were diagnosed with LCIS, resulting in a 20.8% rate of multifocal/multicentric LCIS. In the contralateral breast, one LCIS (2.1%) and seven other malignancies (invasive carcinoma and DCIS, 14.6%) were also diagnosed.
LCIS has been previously reported to involve multifocal or multicentric lesions in the ipsilateral breast in more than half the patients and bilateral LCIS in approximately a third of patients [10-12]. In our study, however, additional LCIS lesions were detected at a significantly lower rate in either breast, even with the use of the latest breast imaging technologies, including digital MMG, USG with Doppler and elasticity imaging, and MRI. This difference may be due to the difference in methods used to evaluate additional lesions. In the study by Rosen et al. [10], 108 of 125 evaluated patients underwent mastectomy for the ipsilateral breast, and 24 had bilateral mastectomy. More extensive examination of the excised breast could have resulted in the detection of additional LCIS lesions that otherwise would have not been detected.
Breast MRI performed in 35 of 48 patients in this study provided limited information beyond MMG and USG for detecting additional LCIS lesions. All 16 additional abnormalities in the ipsilateral breast and 11 in the contralateral breast that were detected on MRI were noted on breast USG. These findings are comparable to several studies that evaluated the use of screening MRI during follow-up for LCIS patients. A study by King et al. [17] showed that there was no additional benefit in cancer detection in women receiving MRI compared to those with annual MMG and breast examinations. Another study by Schwartz et al. [18] demonstrated that screening MRI in women with atypical lesions or LCIS resulted in additional tests in one-fifth of the patients, but with a positive predictive value of only 20%. In a study evaluating the usefulness of MRI in addition to MMG and USG in Korean women with invasive breast cancer, the increase in the detection rate of additional malignancy was only 2.0% for the ipsilateral breast and 0.9% for the contralateral breast compared to 4.6% to 16% in the Western population [19]. When taking these results into account, it is suggested that aggressive evaluation for additional lesions using breast MRI is of limited value in Korean women with LCIS.
There are several limitations to this study. One is the retrospective nature of this investigation. This may have affected the selection of patients, which depended on the review of pathologic reports and imaging findings. Furthermore, the interpretation of the preoperative breast MRI and USG were made by the same radiologist, which raises the concern that the MRI and USG imaging findings may have influenced each other. Another limitation is the small number of patients included in the study. While our study involves a relatively large group of Korean LCIS patients, the number is still insufficient to draw definitive conclusions.
In conclusion, in a modern series of Korean LCIS patients, the incidence of the multifocal/multicentric or bilateral LCIS was lower than that in the historic case series. The role of breast MRI in addition to MMG and breast USG in the detection of additional lesions in LCIS patients had limited value. Further large-scale prospective studies are necessary to evaluate the role of various breast imaging studies in patients with LCIS.
Figure 1. Multifocal/multicentric and bilateral lesions detected on breast ultrasonography (USG) and magnetic resonance imaging (MRI). In the 48 lobular carcinoma in situ (LCIS) patients, additional imaging abnormalities in the ipsilateral breast requiring biopsy were detected in 19 (39.6%) and 16 (45.7%) patients on USG and MRI, respectively. All additional lesions seen on MRI were also visible on USG and were subjected to biopsies. Among them, 10 lesions (52.6%) were LCIS, and the remaining nine were benign lesions. Contralateral imaging abnormalities requiring biopsy were detected in 14 (29.2%) and 11 patients (31.4%) on USG and MRI, respectively. Among them, only one was pure LCIS. Seven lesions were diagnosed as malignant tumors, such as invasive ductal carcinoma (IDC), invasive lobular carcinoma (ILC), and ductal carcinoma in situ (DCIS). In total, 20.8% (10/48) of the patients had ipsilateral LCIS, and 16.7% (8/48) had contralateral malignancies. 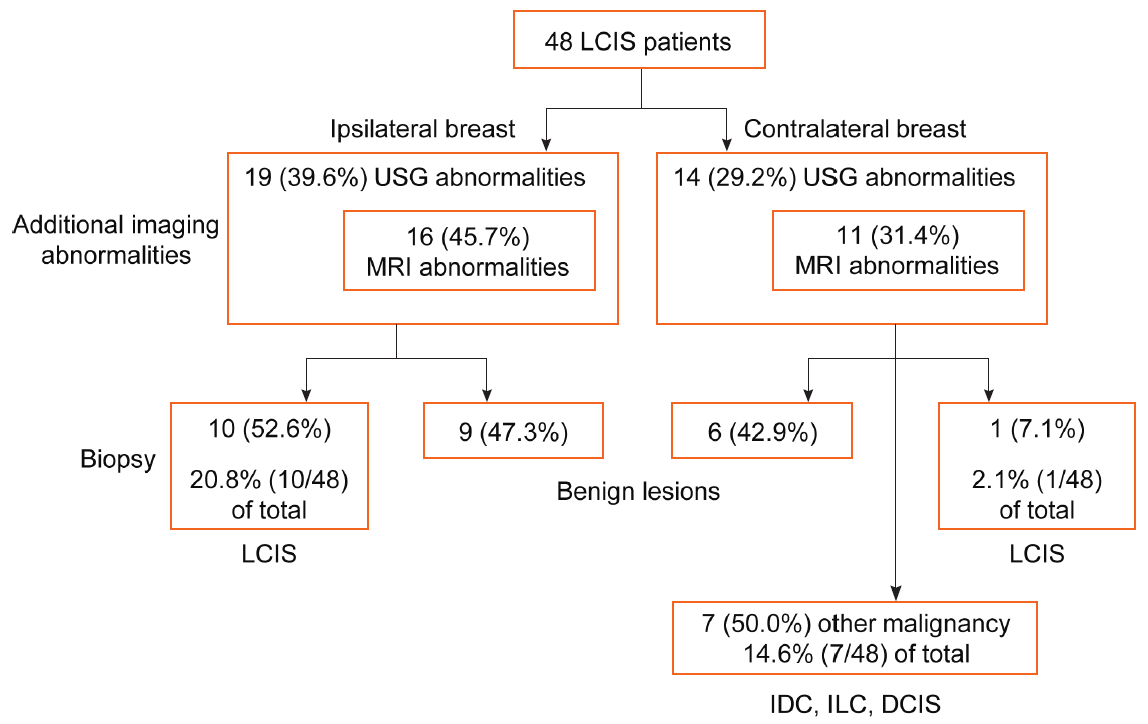
Figure 2.Examples of breast images with additional multicentric and bilateral lesions detected in a patient diagnosed with lobular carcinoma in situ (LCIS). A 50-year-old woman was admitted for LCIS diagnosed with a core needle biopsy of a lesion in the right upper breast. (A) Mammography (MMG) of the ipsilateral breast shows known LCIS in the upper breast and an additional lesion in the lower breast. (B) The same lesions were detected on ultrasonography (USG) and (C) magnetic resonance imaging (MRI). (D) MMG of the contralateral breast shows an additional lesion in the lower breast. (E) The same lesion was detected on USG and (F) MRI. Both additional lesions were diagnosed as LCIS on excisional biopsy. 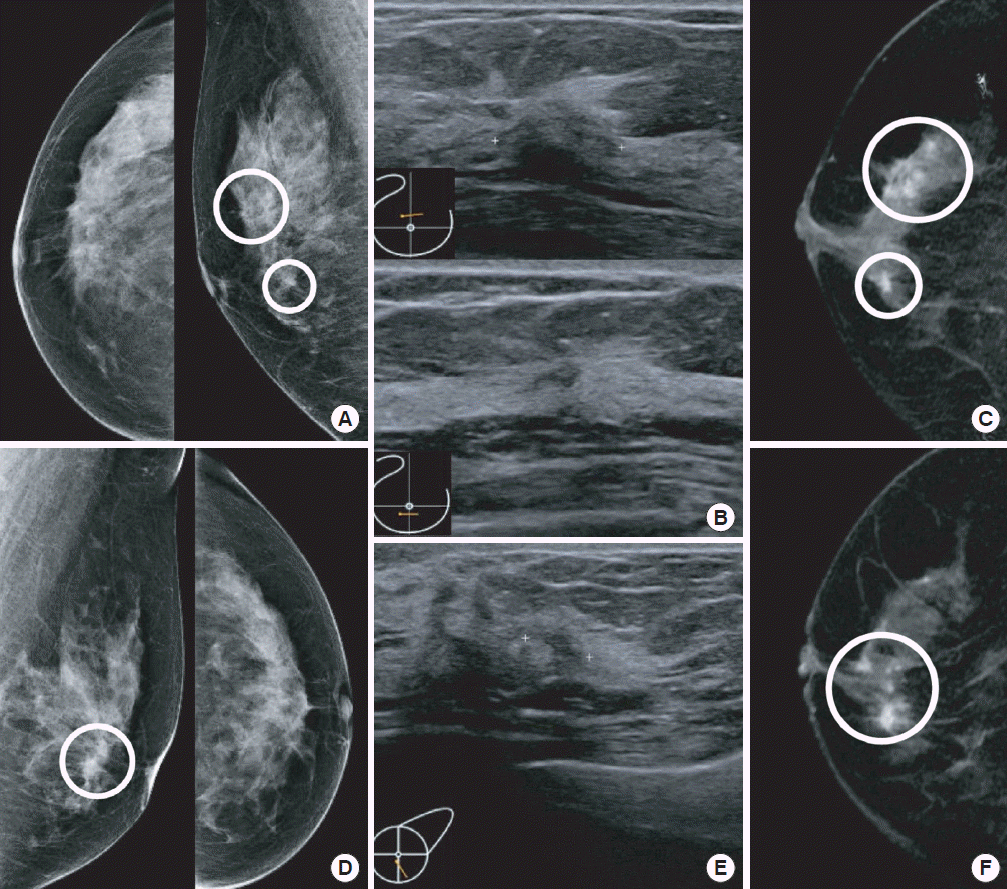
Table 1.Characteristics of 48 pure lobular carcinoma in situ patients
Table 2.Imaging findings of initially biopsied lesions REFERENCES1. Ewing J. Neoplastic Diseases: A Text-book on Tumors. Philadelphia: W.B. Saunders Company; 1919.
2. Foote FW, Stewart FW. Lobular carcinoma in situ: a rare form of mammary cancer. Am J Pathol 1941;17:491-496.3.
3. Haagensen CD, Lane N, Lattes R, Bodian C. Lobular neoplasia (so-called lobular carcinoma in situ) of the breast. Cancer 1978;42:737-69.
4. Dupont WD, Page DL. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med 1985;312:146-51.
5. Page DL, Schuyler PA, Dupont WD, Jensen RA, Plummer WD Jr, Simpson JF. Atypical lobular hyperplasia as a unilateral predictor of breast cancer risk: a retrospective cohort study. Lancet 2003;361:125-9.
7. Hussain M, Cunnick GH. Management of lobular carcinoma insitu and atypical lobular hyperplasia of the breast: a review. Eur J Surg Oncol 2011;37:279-89.
8. Page DL, Kidd TE Jr, Dupont WD, Simpson JF, Rogers LW. Lobular neoplasia of the breast: higher risk for subsequent invasive cancer predicted by more extensive disease. Hum Pathol 1991;22:1232-9.
9. Li CI, Anderson BO, Daling JR, Moe RE. Changing incidence of lobular carcinoma in situ of the breast. Breast Cancer Res Treat 2002;75:259-68.
10. Rosen PP, Braun DW Jr, Lyngholm B, Urban JA, Kinne DW. Lobular carcinoma in situ of the breast: preliminary results of treatment by ipsilateral mastectomy and contralateral breast biopsy. Cancer 1981;47:813-9.
11. Rosen PP, Senie R, Schottenfeld D, Ashikari R. Noninvasive breast carcinoma: frequency of unsuspected invasion and implications for treatment. Ann Surg 1979;189:377-82.
12. Urban JA. Bilaterality of cancer of the breast: biopsy of the opposite breast. Cancer 1967;20:1867-70.
13. Pope TL Jr, Fechner RE, Wilhelm MC, Wanebo HJ, de Paredes ES. Lobular carcinoma in situ of the breast: mammographic features. Radiology 1988;168:63-6.
14. Sonnenfeld MR, Frenna TH, Weidner N, Meyer JE. Lobular carcinoma in situ: mammographic-pathologic correlation of results of needle-directed biopsy. Radiology 1991;181:363-7.
15. Moon HG, Han W, Noh DY. Underweight and breast cancer recurrence and death: a report from the Korean Breast Cancer Society. J Clin Oncol 2009;27:5899-905.
17. King TA, Muhsen S, Patil S, Koslow S, Oskar S, Park A, et al. Is there a role for routine screening MRI in women with LCIS? Breast Cancer Res Treat 2013;142:445-53.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||