AbstractMost local recurrences following mastectomy with flap reconstruction develop in the superficial skin and subcutaneous fat layer or at the mastectomy site. However, there is a possibility of local recurrence in the reconstructed tissue with lymphangiogenesis into the reconstructed flap if the flap was harvested from a distant organ. We herein report a case of a patient with invasive carcinoma that exhibited extensive lymphovascular invasion and tumor emboli at the initial diagnosis and repeatedly recurred not only in the original breast skin and tissue, but also in the reconstructed flap.
INTRODUCTIONTransversus rectus abdominis myocutaneous (TRAM) flap reconstruction after total mastectomy is considered to be an oncologically safe procedure and shows good cosmetic outcomes, even in patients with advanced breast cancer. This reconstructive technique performed after total mastectomy does not increase the incidence of local recurrence because the development of local recurrence depends on the stage and pathologic characteristics of the primary breast cancer [1-3].
The local recurrence rate after mastectomy followed by breast reconstruction is reported to range from 2% to 11% and usually occurs within the first 5 years after surgery [1-3]. Most local recurrences following mastectomy with flap reconstruction develop in the superficial skin and subcutaneous fat layer or at the mastectomy site. Local recurrence in the reconstructed flap itself rarely occurs because the flap is derived from an organ other than the breast [4]. However, there is a possibility of local recurrence in the reconstructed tissue with lymphangiogenesis into the reconstructed flap if the flap was harvested from a distant organ [5,6].
We herein report a case of repeated recurrence of breast cancer arising within the remnant breast skin, chest wall, and reconstructed TRAM flap. The breast cancer had initially been diagnosed without dermal invasion or evidence of inflammation.
CASE REPORTA 42-year-old woman presented with a palpable mass in her right upper central breast in November 2011. She had no specific personal or family history of malignancy. Ultrasonography revealed a 1.7 cm irregular, hypoechogenic mass at the 1 o’clock position in her right breast, 6 cm from the nipple (Figure 1A). Two suspicious axillary lymph nodes were also detected. The breast magnetic resonance imaging and positron emission tomography/computed tomography (PET/CT) findings were concordant with the breast ultrasonography findings, and there was no evidence of distant metastasis on PET/CT.
Needle biopsy confirmed that the breast mass was an invasive ductal carcinoma with multiple foci of lymphovascular invasion. The patient underwent breast-conserving surgery with axillary lymph node dissection because of a positive result on sentinel lymph node biopsy. The final pathologic report indicated that the breast tumor was a 2.0 cm invasive ductal carcinoma with extensive lymphovascular invasion, and five of 15 axillary lymph nodes were confirmed to have metastatic carcinoma. The tumor had been removed with clear resection margins. Immunohistochemical profiling showed negativity for estrogen and progesterone receptors, positivity for the human epidermal growth factor receptor 2 gene, and a Ki-67 index of 30%.
Adjuvant chemotherapy with concurrent trastuzumab treatment and sequential radiotherapy (28 fractions with 5,040 cGy to the remnant breast tissue and five booster fractions with 1,000 cGy to the tumor bed) were administered. However, in November 2012, which was 11 months after the initial breast cancer surgery, multiple skin nodules with erythematous patches developed on the right breast (Figure 1B). Breast ultrasonography and magnetic resonance imaging revealed multiple irregular nodules in the remnant breast; the nodules were pathologically confirmed to be recurrent masses.
Combination treatment with capecitabine and lapatinib was initially considered because the recurrent nodules were too diffuse to remove completely. However, after six cycles of chemotherapy, the tumors showed resistance and spread to the contralateral breast. The patient underwent a salvage mastectomy with a TRAM flap for chest wall reconstruction on the right side and a modified radical mastectomy on the left side in May 2013. The pathologic findings were very similar to those of initial breast cancer. After surgery, palliative chemotherapy (cyclophosphamide, methotrexate, and fluorouracil) was followed by treatment with trastuzumab emtansine. Mastectomy for the left breast cancer and palliative chemotherapy were applied because the authors viewed the left breast cancer as a kind of metastatic disease from the right breast cancer.
After 10 cycles of trastuzumab emtansine, multiple skin nodules with erythema were detected again on the right breast, and imaging showed involvement of the reconstructed TRAM flap (Figures 1C, 2). The chemotherapy could not be continued because of bone marrow suppression. Thus, multicentric, wide excision of each metastatic nodule was performed in June 2014, and a V-Y advancement flap was created for coverage of the chest wall. The pathologic findings were very similar to those of the initial breast cancer and previously recurred cancer. Pathologic findings of both recurred nodules showed multiple foci of lymphovascular invasion, which presented as lymphatic emboli, even if one was detected in the remnant breast tissue and the other was in the reconstructed TRAM flap (Figure 3). Since then, the patient has been receiving combination treatment with lapatinib plus vinorelbine under regular surveillance.
DISCUSSIONSeveral options are available for reconstruction after breast conserving surgery for recurrent breast cancer. Plastic surgeons usually prefer the use of an autologous tissue flap instead of an implant insertion technique. The advantages of autologous tissue flaps include good cosmetic outcomes even after radiotherapy as well as the availability of various surgical scales depending on the type of harvested flap [7].
However, fat necrosis, which may occur after autologous tissue flap reconstruction, is sometimes difficult to differentiate from cancer recurrence. Rarely, local recurrence is detected on a reconstructed flap that was harvested from a distant organ. This occurs not because of lymphatic invasion by tumor emboli, but because the tumor emboli penetrate lymphatic or vascular channels that have newly developed through lymphangiogenesis [8]. Despite complete removal of the primary tumor, circulating tumor cells or emboli can cause a relapse of breast cancer. These circulating tumor cells or emboli may spread to the contralateral breast, and it could result in a metastatic lesion. If the tumor characteristics are similar to those of the primary lesion, a mastectomy would be a good choice, which would reduce the tumor burden.
Whereas sarcomas tend to extend through vascular channels, carcinomas tend to spread through lymphatic channels [5]. Lymphogenic metastasis usually occurs in patients with breast carcinoma, prostate carcinoma, gastrointestinal carcinoma, and melanoma [9]. Physiologic lymphangiogenesis proceeds from embryonic development, wound healing, and the regeneration process. A newly developed lymphatic network can be observed in patients who have undergone organ transplantation or flap reconstruction of the breast. However, tumor-induced lymphangiogenesis may accelerate this process and is a prognostic factor in patients with invasive breast cancer [10].
Lymphovascular invasion and the presence of lymphovascular tumor emboli are also significant prognostic factors in patients with breast cancer because they may lead to local and distant recurrence with poor survival [11,12]. The lymphatic circulation is one of the most important sources of metastasis of breast cancer, and lymphovascular invasion is significantly correlated with lymphangiogenesis [13]. Therefore, an initial pathologic finding of multiple foci of lymphovascular invasion in the primary breast cancer may be an important risk factor for recurrence of the breast cancer in the native breast or reconstructed flap. However, this does not mean that breast conservation or immediate reconstruction would be contraindicated in breast cancer that shows extensive lymphovascular invasion or tumor emboli. If the breast tumor is removed with clear resection margins, breast conserving surgery or immediate breast reconstruction could be a good treatment choice.
The local recurrence rate after mastectomy for breast cancer is about 2% to 10 % with or without reconstruction [14,15]. According to our data, the local recurrence rate after mastectomy was 2.7% in patients who received immediate reconstruction and 2.9% in patients who did not. Breast cancer recurrence after flap reconstruction may occur in the remnant breast skin or tissue, tumor or mastectomy bed, or reconstructed flap [4]. However, physicians may easily overlook the need to extend cancer surveillance beyond the reconstructed flap or mastectomy bed. Breast cancer surveillance should include not only the remnant breast tissue or mastectomy bed, but also the reconstructed flap tissue.
Extensive lymphovascular invasion, tumor emboli, and lymphangiogenesis are all poor prognostic factors in patients with breast cancer. When the primary tumor shows such characteristics, follow-up studies for recurrence should be carried out not only in the mastectomy bed or remnant breast skin, but also in the reconstructed flap tissues. However, the association between local recurrence and lymphangiogenesis in our case is still a circumstantial one. Further investigation would be necessary to demonstrate their association.
Figure 1.Clinical finding of invasive carcinoma on right breast. (A) Primary breast cancer was located on upper inner quadrant of right breast. A breast-conserving surgery had been performed at the initial diagnosis of breast cancer. (B) The first event of local recurrence was found as multiple skin nodules on remnant breast. (C) At second event of local recurrence, the nodules occurred not only in the mastectomy bed and chest wall but also in the reconstructed transversus rectus abdominis myocutaneous flap. 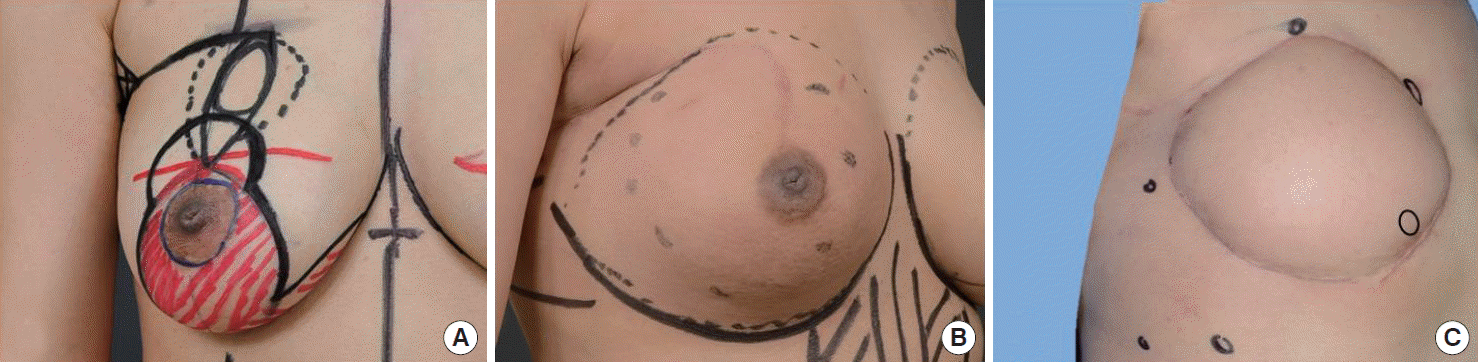
Figure 2.Image findings of local recurrence on reconstructed transversus rectus abdominis myocutaneous (TRAM) flap of right breast. (A) An irregular, hypoechoic mass (arrow) was shown in ultrasonography which was located on TRAM flap (right side of dot line). (B) In magnetic resonance imaging findings, the enhanced, multifocal nodules (arrow) were detected on reconstructed TRAM flap. 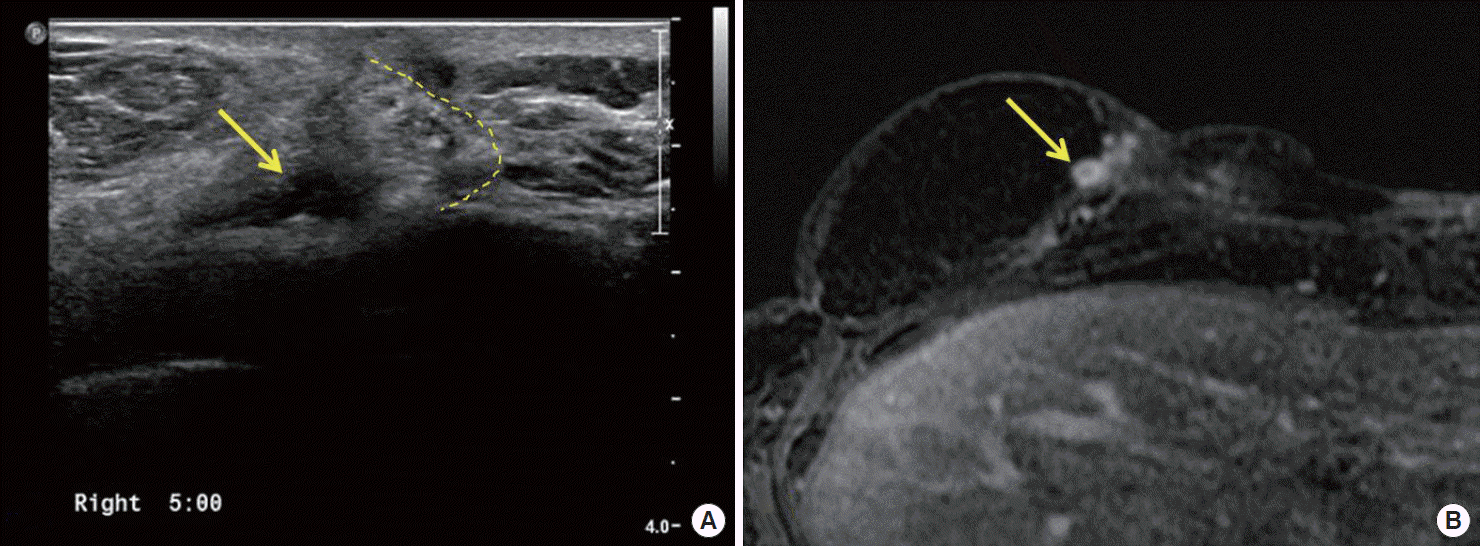
Figure 3.Histopathologic findings of patient with repeatedly recurred breast cancer (H&E stain, ×100). The first and second event of recurred breast cancer show very similar pathologic findings, even if one is detected in remnant breast tissue and the other is in reconstructed transversus rectus abdominis myocutaneous (TRAM) flap. (A) A pathologic finding of the first recurred breast cancer on remnant breast. Multiple foci of lymphovascular invasion are noted in dermis (arrowheads). (B) A pathologic finding of recurred skin nodule which occurred in reconstructed TRAM flap. Multiple lymphatic emboli which are similar features with the first recurrence are noted (arrows). 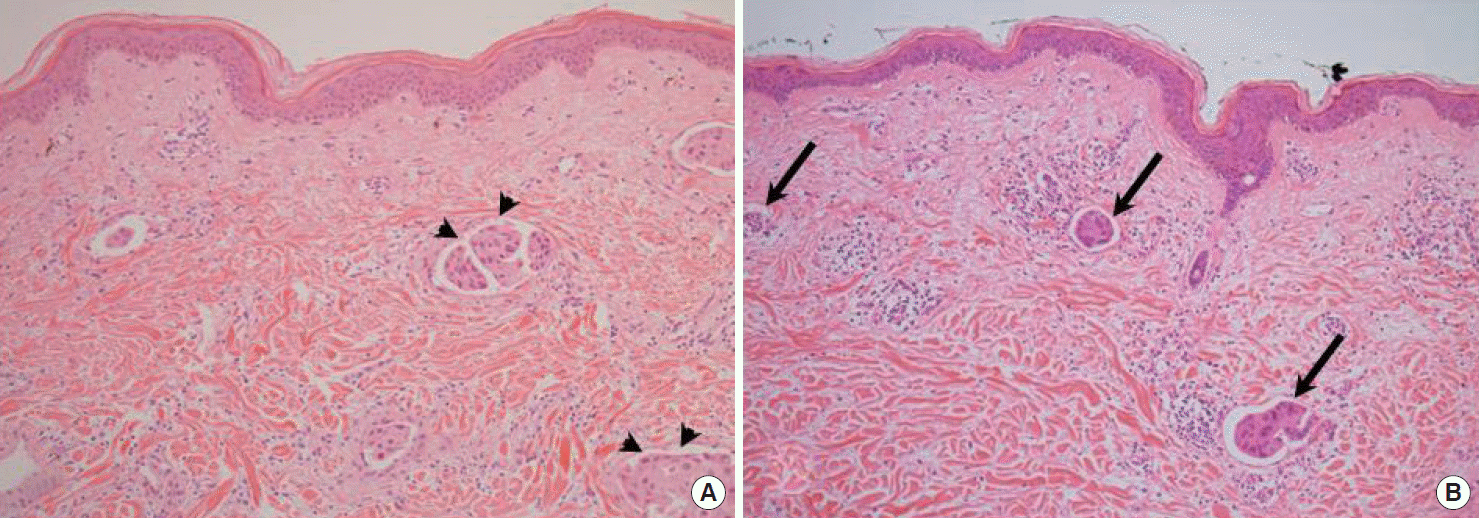
REFERENCES1. Kroll SS, Khoo A, Singletary SE, Ames FC, Wang BG, Reece GP, et al. Local recurrence risk after skin-sparing and conventional mastectomy: a 6-year follow-up. Plast Reconstr Surg 1999;104:421-5.
2. Foster RD, Esserman LJ, Anthony JP, Hwang ES, Do H. Skin-sparing mastectomy and immediate breast reconstruction: a prospective cohort study for the treatment of advanced stages of breast carcinoma. Ann Surg Oncol 2002;9:462-6.
3. Medina-Franco H, Vasconez LO, Fix RJ, Heslin MJ, Beenken SW, Bland KI, et al. Factors associated with local recurrence after skin-sparing mastectomy and immediate breast reconstruction for invasive breast cancer. Ann Surg 2002;235:814-9.
4. Yoo H, Kim BH, Kim HH, Cha JH, Shin HJ, Lee TJ. Local recurrence of breast cancer in reconstructed breasts using TRAM flap after skin-sparing mastectomy: clinical and imaging features. Eur Radiol 2014;24:2220-6.
5. Wilting J, Hawighorst T, Hecht M, Christ B, Papoutsi M. Development of lymphatic vessels: tumour lymphangiogenesis and lymphatic invasion. Curr Med Chem 2005;12:3043-53.
6. Ran S, Volk L, Hall K, Flister MJ. Lymphangiogenesis and lymphatic metastasis in breast cancer. Pathophysiology 2010;17:229-51.
7. Clough KB, Kaufman GJ, Nos C, Buccimazza I, Sarfati IM. Improving breast cancer surgery: a classification and quadrant per quadrant atlas for oncoplastic surgery. Ann Surg Oncol 2010;17:1375-91.
8. Mahooti S, Porter K, Alpaugh ML, Ye Y, Xiao Y, Jones S, et al. Breast carcinomatous tumoral emboli can result from encircling lymphovasculogenesis rather than lymphovascular invasion. Oncotarget 2010;1:131-47.
9. Kaiserling E. Metastasis of malignant tumors. In: Földi M, Földi E, editors. Földi’s Textbook of Lymphology. Munich: Urban and Fischer; 2003. p 455-65. Cited from Wilting J, Hawighorst T, Hecht M, Christ B, Papoutsi M. Development of lymphatic vessels: tumour lymphangiogenesis and lymphatic invasion. Curr Med Chem 2005;12:3043-53.
10. Schoppmann SF, Bayer G, Aumayr K, Taucher S, Geleff S, Rudas M, et al. Prognostic value of lymphangiogenesis and lymphovascular invasion in invasive breast cancer. Ann Surg 2004;240:306-12.
11. Mohammed ZM, McMillan DC, Edwards J, Mallon E, Doughty JC, Orange C, et al. The relationship between lymphovascular invasion and angiogenesis, hormone receptors, cell proliferation and survival in patients with primary operable invasive ductal breast cancer. BMC Clin Pathol 2013;13:31.
12. Dinshaw KA, Budrukkar AN, Chinoy RF, Sarin R, Badwe R, Hawaldar R, et al. Profile of prognostic factors in 1022 Indian women with early-stage breast cancer treated with breast-conserving therapy. Int J Radiat Oncol Biol Phys 2005;63:1132-41.
13. Song YJ, Shin SH, Cho JS, Park MH, Yoon JH, Jegal YJ. The role of lymphovascular invasion as a prognostic factor in patients with lymph node-positive operable invasive breast cancer. J Breast Cancer 2011;14:198-203.
|
|
||||||||||||||||||||||||||||||||||