INTRODUCTION
Breast cancer encompasses a heterogeneous population of tumors characterized by a variety of clinical, pathological, and molecular features [1-3]. The traditional division of breast cancers into hormone receptor positive and negative helps to guide patient management. Gene expression profiling has classified breast tumors into five major molecular subtypes with different outcomes [4]. Molecular markers such as estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) have been used to classify patients into different subtypes. Recently, gene expression studies using DNA microarrays have identified five common subtypes of breast cancer: luminal A, luminal B, HER2 overexpression, basal-like and normal breast-like tumor [1,4-6].
However, a subgroup of hormone receptor-positive patients relapse irrespective of standard hormonal therapy. The two luminal subtypes, A and B, all express the ER or PR receptor genes. Additionally, the ER+ or PR+ luminal types in breast cancer confer a better prognosis but less chemosensitivity. Compared with luminal A, luminal B tumors have a poor prognosis [4,7,8].
Luminal A tumors resemble luminal B tumors; they are distinguished only because the lists of genes used in gene expression analyses to identify subtypes are not accurate enough and because luminality reflects a continuum from poorly differentiated and highly proliferative (luminal B) to well-differentiated and less proliferative (luminal A). The prognostic distinction between luminal A and B suggest that grade and p53 are also involved. Mutation of p53 is generally associated with basal breast cancer. However, we demonstrated its impact in luminal cases [9]. In another study, p53 expression observed in BRCA1 luminal cases corresponded to a true mutation in only four of seven cases [10]. This suggests that p53 expression could be associated with proliferation in luminal cases independent of mutation.
Prognostic factors are important for clinical decision making and for tailoring treatment of individual patients with cancer. Axillary lymph node (ALN) status is the single most reliable prognostic indicator of early breast cancer, followed by primary tumor size [11].
The aim of our study was to identify the prognostic factors of patients with breast cancer by luminal type among clinicopathological and immunohistochemical factors.
METHODS
Patients
Between January 1994 and October 2000, 405 patients with pathologic stage I-III breast cancer according to the American Joint Committee on Cancer stages were treated with curative surgery without neoadjuvant chemotherapy at Seoul St. Mary’s Hospital. All patients had available ER, PR, and HER2 receptor status. Medical records were retrospectively reviewed to find clinicopathological features such as menopausal status, tumor size, lymph node stage, operation type, histologic grade, status of chemotherapy or radiation therapy, status of Ki-67 and p53 expression, site and date of first recurrence, and survival data in patients with breast cancer who received surgery. Tumors were all invasive ductal carcinomas. Stage IV or nonluminal type breast cancers were excluded. Unknown histology was also excluded. The number of eligible patients was 215. This study was approved by institutional review board. (Approval No. KC11RISI0517)
Molecular subtypes
Breast cancer molecular subtypes were defined as luminal A (ER/PR+, HER2-), luminal B (ER/PR+, HER2+), HER2-enriched (HER2+, ER-, and PR-), and triple-negative (HER2-, ER-, and PR-) subgroup. In this study, patients were classified two groups according to the receptor status: luminal A (ER/PR+, HER2-), luminal B (ER/PR+, HER2+). ER and PR status were reviewed by medical records, and hormone receptor status was determined using a ligand binding assay. A result exceeding 10 fmol/mg was considered positive for the presence of the particular receptor. Tissue microarray of primary breast tissue was used for analysis of HER2 overexpression. Positive HER2 was determined using either immunohistochemistry (IHC) 3+ staining or amplification on fluorescence in situ hybridization. For each antibody, a sample was considered as positive when the quick score was strictly superior to 0. However, the Ki-67 status was expressed in terms of percentage of positive cells, with a cutoff of 14% of positive cells, which was the mean value of available databases in this study.
Recurrence pattern and survival analysis
The pattern of the recurrence site was categorized as local recurrence; regional recurrence including ipsilateral axillary, supraclavicular, or internal mammary lymph node; contralateral recurrence; and distant metastasis. Distant metastasis was defined as a recurrence of breast cancer developing beyond the ipsilateral or contralateral breast, chest wall, or regional lymph node including ipsilateral axillary, supraclavicular, or internal mammary lymph node. The median follow-up duration, calculated from the date of surgery, was 79 months (range, 5-147 months). The endpoints of the study were locoregional or distant recurrence as the first event, overall survival (OS), and recurrence-free survival (RFS). OS was defined as the time from the date of surgery to death from any cause, and RFS was defined as the time from the date of surgery to relapse or death.
Statistical analyses and ethics
SPSS version 11.0 (SPSS Inc., Chicago, USA) was used for statistical analysis. Student t-test was used to calculate and compare the mean values in continuous variables and the results are given as mean±standard deviation. Cox proportional hazards regression analysis was used for evaluating risk factors for OS and RFS. Kaplan-Meier analyses for OS and RFS was accessed for survival analysis, and the generalized Wilcoxon test was used for estimating the difference of survival among subtypes. A p value <0.05 was considered statistically significant. The present study was approved by the Institutional Review Board of the Seoul St. Mary’s Hospital, The Catholic University of Korea.
RESULTS
Patient characteristics
From January 1994 to October 2000, 405 patients were diagnosed with breast cancer. Patients with known distant metastases were excluded. Among the 215 eligible patients who showed positive immunoreactivity for ER or PR, 40 patients (18.4%) showed positive immunoreactivity for HER2. As a result, 175 (81.6%) and 40 patients were classified into the luminal A and luminal B groups, respectively. The mean age of patients was 47.8±10.7 years (range, 26-80 years). The median follow-up period was 88 months (range, 2-164 months). All eligible patients underwent breast-conserving surgery and postoper-ative whole breast radiotherapy or total mastectomy. The characteristics of the patients with breast cancer by luminal type are outlined in Table 1. The luminal B subtype occurred more frequently than the luminal A subtype in patients who received chemotherapy (p=0.03) and were more frequent associated with a higher p53 positive rate (p= 0.01). However, there were not significant differences in the distribution of Ki-67 positive rate (p=0.72), and treated radiation and operative type (p=0.94 and p=0.09, respectively). The distributions of tumor size and the nodal status, overall pathologic stage, and menopausal status were not different between the luminal A and luminal B subtypes.
Pattern of recurrence
During the follow-up period, there were 21 (9.3%) and 44 (21.4%) patients who experienced locoregional recurrence and distant metastasis, respectively, as the first site of recurrence (p=0.10) (Table 2). Median time to locoregional recurrence or distant metastasis was 40 months (range, 1-135 months). Distant metastasis as the first site of recurrence occurred in 44 patients (21.4%) with the luminal subtype after a median time of 48 months. The overall incidence of distant recurrence during the follow-up period was 18.9% and 25% in the luminal A and luminal B subtypes, respectively (p=0.27).
Prognostic factor for RFS and OS in luminal type breast cancer
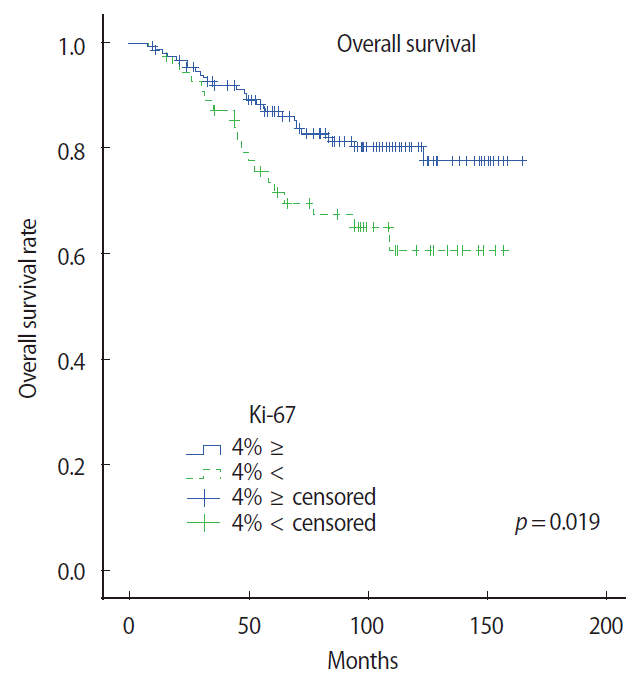
We studied the impact of nine histoclinical and immunohistochemical factors on RFS and OS. Univariate analysis showed that pathological tumor stage, pathological ALN status, and Ki-67 expression were prognostic factors of RFS and OS. However, multivariate Cox proportional hazard models indicated that RFS and OS were significantly related to the number of involved ALNs. Additionally, pathological tumor stage was associated with RFS in the multivariate analysis (Tables 3, 4). The 10-year OS and RFS rates of all patients were 75.4% and 69.7%, respectively. The OS rate according to the Ki-67 expression in the luminal type group was significantly different between the two subgroups (p=0.012) (Figure 1).
Prognostic factor for RFS and OS in luminal A subtype breast cancer
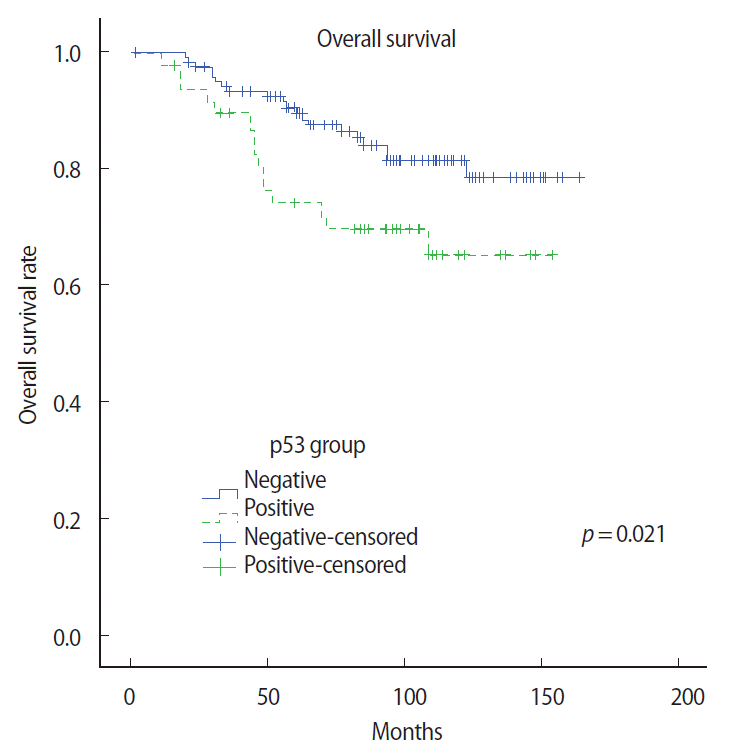
To study subgroup analysis, the 10-year OS rate of each subtype was 78.6% and 62.4%, respectively. The hazard ratio of the luminal A and B subtypes were 1.63 (95% confidence interval [CI] 0.46-5.73, p= 0.4461) and 2.43 (95% CI 1.44-4.09, p=0.0009), respectively. Especially, the luminal A subgroup showed interesting results. Univariate analysis showed that pathological tumor stage and pathological ALN status were prognostic factors of RFS and OS. However, Ki-67 expression and p53 expression were associated with OS only in the univariate analysis (p=0.015 and p=0.039, respectively). Additionally, a multivariate Cox proportional hazard model indicated that RFS and OS were significantly related to the number of involved ALNs (Tables 5 and 6). OS rate according to the Ki-67 expression and p53 expression in the luminal A subtype group was significantly different between the two subgroups (p=0.019 and p=0.021, respectively) (Figures 2 and 3).
DISCUSSION
The clinical characteristics of the patients were remarkable in terms of the high proportion of young premenopausal patients. The median age of the studied patients was far below 50 years because breast cancer in Korean women tends to occur earlier than in Western countries, showing a peak incidence at ages 40 to 49 years [12]. In general, Korean women who have breast tumors >3 cm are not candidates for breast-conserving operations because they have smaller breasts than those of women from Western countries. In this study, patients who have breast tumors larger than T2 (>2 cm) constituted two-thirds of the patients. Therefore, many patients were inclined to undergo mastectomy. In our study, based on a review of data with a long follow-up, the tumor size and the absolute number of metastatic ALNs identified at pathological staging were independent predictors of both RFS and OS.
Grossly, the 215 subtypes showed a similar distribution of subtypes as found in previous studies [7,13]. Our series contained 19% of HER2+ cases, which is within a published range of 17% to 37%. The 81% frequency of luminal A was high and the proportion of luminal B was low. In this series, the 215 patients with surgically-treated breast cancer were evaluated based on ER, PR, and HER2 expression status. This study was limited due to its retrospective analysis, relatively small sample size in the luminal A and B subgroups, and potential existence of other variables [6]. Because no patient received trastuzumab therapy, the results could be changed by trastuzumab therapy. During the follow-up period, overall incidence of locoregional and distant recurrence as first site of recurrence was not significantly different between subgroups. Although not statistically significant, the time to recurrence in the luminal B subtype was shorter than that of other subtype.
Univariate analysis identified four factors: tumor size, axillary lymph node status, p53 status, and Ki-67 status. We showed the prognostic impact of p53 in two previous studies of luminal cases [9,14]. Ki-67 expression higher than 20% is one of the parameters able to distinguish luminal A from luminal B [15], but its specific prognostic impact in luminal cases had not been described. An important question is whether the combination of p53 and Ki-67 predicts pure prognosis. Few studies using profiling of ER+ breast cancers have established a signature able to predict the prognosis. The oncotype DX recurrence score [16] is a commercially available assay (Genomic Health, Redwood City, USA) that predicts recurrence in ER+ cases. It is a polymerase chain reaction-based assay on paraffin-embedded tissue using 16 cancer-related genes and 5 controls. The histopronostic grade and recurrence score were significant. Only three of the 16 genes are common with the factors we tested here (ER, PR, and Ki-67). IHC was used to establish a multiparametric score in ER+ cases on a series of 257 ER+ cases treated by tamoxifen; a multimarker model was established from nine markers and five of them were retained in a mathematic model: ER, PR, p53, erbB-2, and Ki-67. This model was more prognostic than the Nottingham prognostic index [14].
A study of a series of 140 cases used 23 antibodies and identified a prognostic score for ER+ breast cancer without any notion of hormonal therapy [17]. Five factors were retained by Cox analysis (p53, NDRG1, CEACAM5, SLC7A5, and HTF9c), but regression tree analysis retained six factors (p53, PR, Ki-67, NAT1, SLC7A5, and HTF9c). The best hazard ratio (HR) was obtain by the Cox model (HR, 2.21; p=0.0008).
p53 and Ki-67 are the two factors common with our series. This again underlines the impact of proliferation in luminal cases. How-ever, our analysis, with two factors, could be an easier means to study ER+ cases.
In summary, Ki-67 expression was an important prognostic factor in terms of both RFS and OS in patients with luminal type breast cancer. In addition, study subgroup analysis revealed that expressions of Ki-67 as well as p53 are reliable OS prognostic factors. Therefore, more intensive adjuvant treatment such as chemotherapy will improve the OS in patients with a luminal A subtype and expression of Ki-67 or p53.