AbstractPurposeThe standard care for patients with ipsilateral breast tumor recurrence (IBTR) after breast-conserving surgery (BCS) is a total mastectomy (TM); however, there is growing interest in repeating BCS for IBTR.
MethodsWe retrospectively analyzed patients with IBTR who underwent initial BCS for breast cancer at our institution between January 2000 and December 2018. The Kaplan-Meier method was used to compare survival rates between the standard BCS-TM treatment group and the repeat-BCS group.
ResultsWe enrolled 209 IBTR patients with a median follow-up of 102.3 months. No significant differences were observed in overall survival (10 years: 87.3% vs. 78.8%; hazard ratio [HR], 1.11; 95% confidence interval [CI], 0.44-2.81; p=0.821), distant metastasis free survival (10 years: 73.9% vs. 77.7%; HR, 0.80; 95% CI, 0.37-1.72; p=0.727) and disease-free survival (10 years: 57.1% vs. 65.2%; HR, 0.63; 95% CI, 0.35-1.12; p=0.115) between two groups. Repeat-BCS group showed significantly poorer locoregional recurrence free survival rate than did the TM group (HR, 2.44; 95% CI, 1.06-5.56; p=0.029) but the significance was not shown after excluding ipsilateral breast tumor recurrence events.
INTRODUCTIONBreast cancer is the most commonly diagnosed cancer and the leading cause of cancer-related deaths in women worldwide [1]. The standard surgical treatment for early stage breast cancer is either breast-conserving surgery (BCS) or total mastectomy (TM) with surgical axillary staging. According to the National Surgical Adjuvant Breast and Bowel Project B-06 trial, there were no significant differences in survival outcomes between BCS and TM [2].
Despite improvements in early diagnosis and adjuvant therapies [3], ipsilateral breast tumor recurrence (IBTR) occurs in a substantial proportion of early breast cancer patients. According to the National Comprehensive Cancer Network guidelines, the standard treatment for IBTR after BCS followed by breast irradiation is TM with axillary lymph node staging if level I/II axillary lymph node dissection was not conducted previously [4]. However, BCS has been considered as an alternative treatment for IBTR for patients’ satisfaction with cosmetic outcome, psychosocial effects, and lower complication rates compared to TM [5,6].
Several previous studies had already reported the survival outcomes of surgical options for IBTR after BCS [7-9]. In this study, we retrospectively reviewed and re-investigated the survival outcomes of patients who were diagnosed with IBTR after initial treatment for breast cancer in a single institution to investigate the oncologic safety of repeating BCS after IBTR.
METHODSPatientsThis study was approved by the Institutional Review Board of Seoul National University Hospital (IRB No. 2103-185-1208). The requirement for informed consent was waived for all patients. We collected clinicopathologic data of patients who developed IBTR after BCS between January 2010 and December 2018 from the database of the Seoul National University Hospital Breast Care Center. Those who declined surgical treatment for IBTR and who developed regional recurrence or distant metastasis (DM) before IBTR were excluded. Breast cancer was pathologically staged according to the 8th American Joint Committee on Cancer staging criteria. A total of 209 patients were included in this study.
One of the concerns of repeated BCS is the toxicity of repeated radiotherapy (RT). In this study, all patients who underwent repeat BCS had conducted partial breast irradiation, such as intensity-modulated radiotherapy or volumetric-modulated arc therapy, rather than whole-breast irradiation.
Definition of recurrenceIBTR was defined as the first recurrence (before any systemic or regional lymph node recurrences) in any quadrant of the ipsilateral breast after BCS. Skin metastases were not included in IBTR. Locoregional recurrence (LRR) included the first recurrence in the ipsilateral chest wall, mastectomy scar, skin of the ipsilateral breast, and ipsilateral regional lymph nodes. Recurrence-free survival was defined as the interval between the date of surgical treatment and the date of pathologic confirmation or radiologic diagnosis of any recurrence. When calculating the LRR-free survival (LRRFS), patients with DM were treated as censored at the time of recurrence regarding the competing risk. Disease-free survival (DFS) based upon any type of disease recurrence, and overall survival (OS) was based upon death from any cause.
Statistical analysesDemographic and clinicopathologic factors were compared using the Pearson’s chi-squared test. Log-rank and Breslow tests were used to analyze survival curves derived using the Kaplan-Meier method. Cox proportional hazards regression with forward selection was conducted to adjust for other variables affecting DFS. Statistical significance was set at p< 0.05. All analyses were performed using SPSS (version 25.0; IBM Corp., Armonk, USA) and curves for figures were drawn using GraphPad Prism (version 8.0; GraphPad Software, San Diego, USA).
RESULTSPatient demographics and characteristicsAmong 209 female breast cancer patients, 163 patients (78.0%) received standard TM treatment, and 46 patients (22.0%) received repeat-BCS for IBTR. The median follow-up time for all patients was 102.3 months (range, 5.5-235.3 months), and the median age of the study population at initial diagnosis was 43.7 (range, 20-92 years). More than half of the patients underwent secondary surgery for IBTR within 5 years of the primary surgery. The clinicopathologic characteristics of the initial and secondary treatments are shown in Table 1. For the initial treatment, the initial pT stage was significantly different between the TM and repeat-BCS groups (p = 0.033). Additionally, more patients in the repeat-BCS group underwent RT for IBTR than those in the TM group (p< 0.001).
Relationship between surgical procedures and locoregional recurrenceMedian follow-up time of the TM and repeat-BCS groups were 96.4 months (range, 24.6-224.4) and 128.7 months (range, 24.1-230.5), respectively. During the follow-up period, 10 patients in the repeat-BCS group and 14 patients in the TM group showed LRR events. On univariate analysis, repeat-BCS group showed significantly poorer LRRFS than TM group (log-rank test p= 0.029, Breslow p= 0.171; hazard ratio [HR], 2.44; 95% confidence interval [CI], 1.06-5.56) (Figure 1A). Especially, as patients in the TM group did not show IBTR after secondary operation, we further analyzed after excluding five patients who showed secondary IBTR in the repeat-BCS. Both log-rank and Breslow tests showed no significant difference in the LRRFS between the two groups (log-rank test p= 0.658; HR, 0.79; 95% CI, 0.28-2.26).
Relationship between surgical procedures and other types of recurrenceKaplan-Meier curves of DM-free survival (DMFS), DFS, and OS between the two groups are shown in Figure 1B-D. DMFS, DFS, and OS ten years after surgery were 73.9%, 57.1%, and 87.3% in the repeat-BCS group, while they were 77.7%, 65.2%, and 78.8% in the TM group, respectively. Both log-rank and Breslow tests revealed no significant difference in DMFS, DFS, and OS between the two groups.
On univariate analysis, N stage, lymphovascular invasion, and Ki-67 index of the primary tumor were significantly associated with DFS. Furthermore, age at secondary operation, interval between secondary and primary surgery, T/N stage, histologic grade, lymphovascular invasion, subtypes of secondary tumor, and administration of chemotherapy after secondary surgery were identified as factors affecting DFS. After adjusting for the aforementioned variables, high histologic grade, presence of lymphovascular invasion, and positive axillary nodes at secondary surgery remained significantly associated with DFS. In contrast, the clinicopathologic factors of the primary surgery did not affect DFS after secondary surgery (Table 2).
DISCUSSIONThe current study aimed to compare the long-term survival outcomes of repeating BCS compared to mastectomy in the patients who initially underwent BCS. Although the IBTR rate was higher for patients in the repeat-BCS group, the OS rate of these patients was comparable with those of the patients in the standard TM group. Our results indicate that BCS is an acceptable surgical option for IBTR after initial BCS.
Our results are consistent with comparable retrospective studies addressing this issue. Recent study by Kolben et al. [7] reported no benefit of survival outcomes including DFS and OS in the mastectomy group compared with the repeat-BCS group. Furthermore, Baek et al. [8] conducted a randomized controlled trial including 335 patients that showed no significant differences in both OS (HR, 1.08, 95% CI, 0.49-2.39) and cancer-specific survival (HR, 0.83; 95% CI, 0.35-1.95) between the two groups. Conversely, Chen et al. [9] reported a significant superiority of mastectomy over repeat-BCS. Among 747 patients, 24.0% underwent lumpectomy and 5-year survival rates were 67.0% and 78.0% for the repeat-BCS group and mastectomy group, respectively (p= 0.030). However, only 21.0% of patients in repeat-BCS group had administered radiation treatment for repeat-BCS group which might have affect the prognosis of patients.
Kolben et al. [7] reported no significant difference in LRRFS between the two groups including all patients (p= 0.547) contrary to our results that showed poorer LRRFS in the repeat-BCS group compared to the TM group. However, repeat-BCS had a tendency of poorer LRRFS than TM, and the different result from our study may be due to the relatively small number of patients in repeat-BCS group compared to TM group in both studies. Alpert et al. [10] also reported that two out of 30 patients in repeat-BCS group developed secondary IBTR, resulting in higher IBTR rate than that of TM group. Komoike et al. [11] also reported that 9 of 30 patients of secondary IBTR in repeat-BCS group in 3 years without accessible data on TM group. Thus, shared decision-making with patients and explanation of the chance of secondary IBTR would be crucial in the process of determining the surgical strategy for IBTR.
In addition, another systematic review by Walstra et al. [12] suggested that repeat-BCS followed by re-irradiation—whole breast or partial breast—seems to be a feasible alternative to the surgical option for mastectomy after IBTR. In this study, 30.4% of patients in the repeat-BCS group underwent RT for IBTR, comparable to other studies [7,13]. However, a subgroup analysis according to the administration of secondary RT could not be conducted due to the small number of patients. From the point of view that RT is one of the significant factors affecting recurrence after BCS, further studies would be needed for understanding the role of RT after IBTR [2,14].
This study has several limitations. First, it was a sin center retrospective cohort study with a small sample size. Additionally, we did not collect detailed information on the regimen of adjuvant treatments and the results of genetic tests, including BRCA, which are known to affect the survival of breast cancer patients. Alpert et al. [10] has reported that repeat-BCS is a feasible surgical option only for selected patients, such as those with favorable tumor biology, diagnosed with IBTR early, and not carrying BRCA 1/2 mutations. Furthermore, more than half of the patients did not receive RT, so the effect of RT could not be analyzed. Finally, the event of IBTR in the current study was indistinguishable as either a new primary tumor or a recurrent tumor, which may have raised the selection bias.
Despite these limitations, our study demonstrated that repeat-BCS for IBTR does not show poorer DMFS, DFS and OS rate compared to TM. We propose that repeat-BCS may be a feasible option for IBTR after BCS instead of mastectomy after considering the risk of ipsilateral breast tumor recurrence.
Figure 1.Kaplan-Meier curves showing the survival outcomes between the two surgical options. The Kaplan-Meier curve shows the difference between repeat-BCS and mastectomy groups in LRRFS (A), DMFS (B), DFS (C), and OS (D). The hazard ratio was calculated via a univariate Cox regression analysis. LRRFS = locoregional recurrence free survival; DMFS = distant metastasis-free survival rate; DFS = disease-free survival rate; OS = overall survival; BCS = breast conserving surgery; CI = confidence interval. 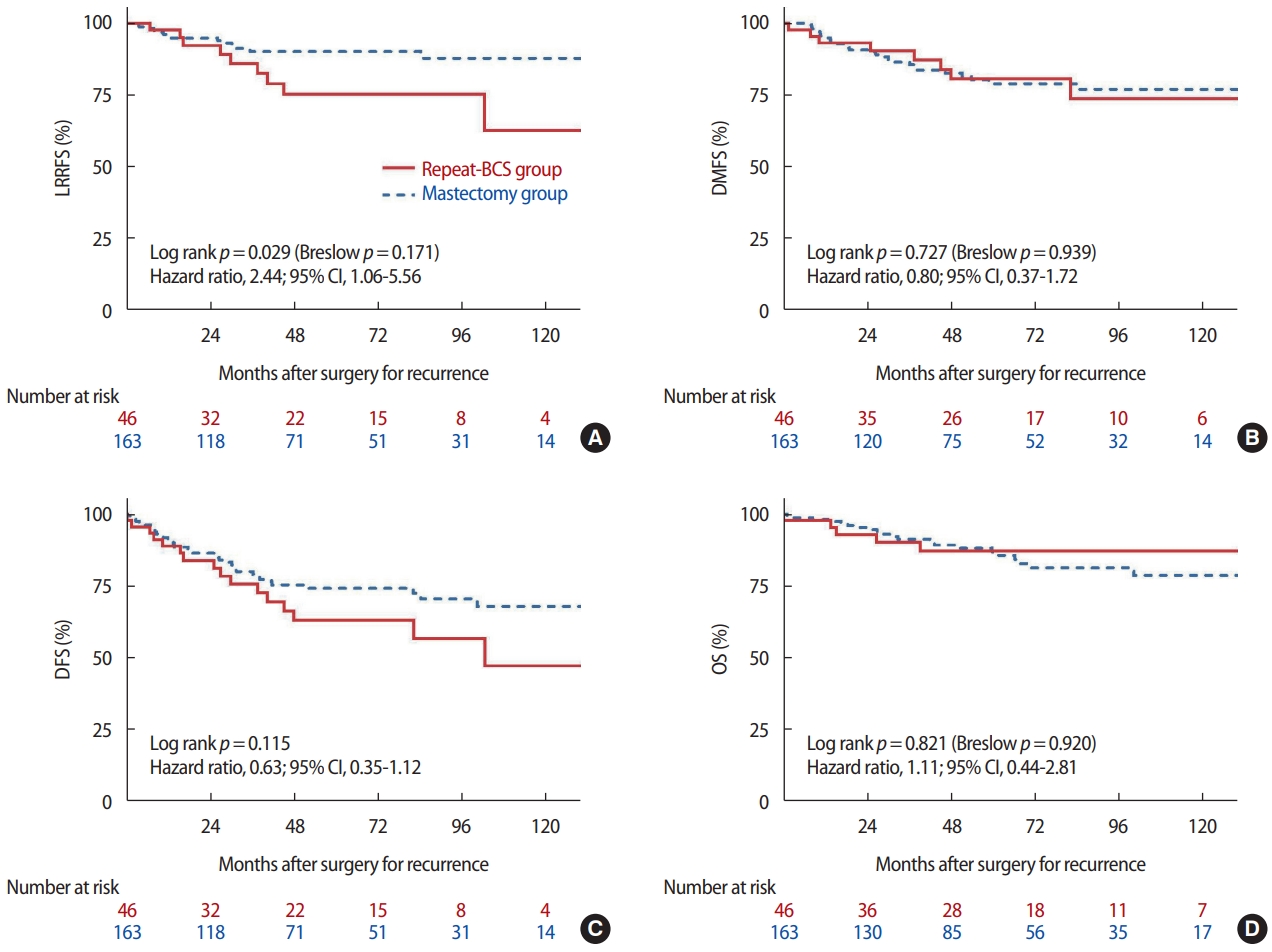
Table 1.Clinical characteristics of patients according to type of surgery for recurrence
Table 2.Univariate and multivariate analyses for disease free survival
REFERENCES1. Cardoso F, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rubio IT, et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2019;30:1194-220.
2. Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002;347:1233-41.
3. Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med 2005;353:1784-92.
4. Breast Cancer (Version 8.2021). National Comprehensive Cancer Network. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. 2021. Accessed October 9th, 2021.
5. Corradini S, Reitz D, Pazos M, Schönecker S, Braun M, Harbeck N, et al. Mastectomy or breast-conserving therapy for early breast cancer in real-life clinical practice: outcome comparison of 7565 Cases. Cancers 2019;11:160.
6. Fujishiro S, Mitsumori M, Kokubo M, Nagata Y, Sasai K, Mise K, et al. Cosmetic results and complications after breast conserving therapy for early breast cancer. Breast Cancer 2000;7:57-63.
7. Kolben T, Schwarz TM, Goess C, Blume C, Degenhardt T, Engel J, et al. Surgical management of ipsilateral breast tumor recurrence. Int J Surg 2015;23:141-6.
8. Baek SY, Kim J, Chung IY, Ko BS, Kim HJ, Lee JW, et al. Long-term survival outcomes of repeat lumpectomy for ipsilateral breast tumor recurrence: a propensity score-matched analysis. Breast Cancer Res Treat 2021;185:155-64.
9. Chen SL, Martinez SR. The survival impact of the choice of surgical procedure after ipsilateral breast cancer recurrence. Am J Surg 2008;196:495-0.
10. Alpert TE, Kuerer HM, Arthur DW, Lannin DR, Haffty BG. Ipsilateral breast tumor recurrence after breast conservation therapy: outcomes of salvage mastectomy vs. salvage breast-conserving surgery and prognostic factors for salvage breast preservation. Int J Radiat Oncol 2005;63:845-51.
11. Komoike Y, Motomura K, Inaji H, Kasugai T, Koyama H. Repeat lumpectomy for patients with ipsilateral breast tumor recurrence after breast-conserving surgery. Oncology 2003;64:1-6.
12. Walstra CJEF, Schipper RJ, Poodt IGM, van Riet, Voogd AC, van der, et al. Repeat breast-conserving therapy for ipsilateral breast cancer recurrence: a systematic review. Eur J Surg Oncol 2019;45:1317-27.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||