AbstractPurposeBreast conserving surgery (BCS) is generally not considered for breast cancer because of concerns about the poor prognosis of triple negative breast cancer (TNBC). We assessed the outcomes of BCS and mastectomy for patients with stage II-IIIA TNBC.
MethodsThe data of 172 breast cancer patients diagnosed with stage II-IIIA TNBC who underwent treatment at Pusan National University Hospital and Pusan National University Yangsan Hospital from 2010 to 2014 were retrospectively analyzed. The patients were divided into the following two groups: patients who underwent BCS (n=101) and those who underwent mastectomy (n=71). The Cox regression model was used to examine the outcomes of both treatments. The median follow-up period was 71 months in the BCS group, and 67 months in the mastectomy group.
ResultsThe median age of the 172 patients was 51 years (range, 22-82 years). In the BCS group, radiation therapy and chemotherapy (p<0.001 and p=0.007, respectively) were performed more frequently. The BCS group had more patients with a high Ki-67 index (p=0.006), while the mastectomy group included more patients with a higher pathologic T (pT) stage (p=0.005). The 5-year loco-regional recurrence-free, disease-free, and overall survival rates of the BCS group versus the mastectomy group were 93.8% versus 95.3%, 89.8% versus 90.7%, and 90.8% versus 86.3%, respectively, but the differences were not statistically significant. Lymphovascular invasion was a risk factor for disease-free survival and advanced stage was an important risk factor for overall survival.
INTRODUCTIONNeoadjuvant chemotherapy increases the possibility of breast conserving surgery (BCS) for locally advanced breast cancer [1-4]. In prospective study in Korea, the conversion rate from mastectomy to BCS was 40.5% in triple negative breast cancer (TNBC) [4]. However, patients who required neoadjuvant chemotherapy had an anxiety or depression disorder because of long periods of treatment before surgery, and side effects such as alopecia, nausea, and diarrhea [5]. Although neoadjuvant chemotherapy should be considered as the first option for advanced TNBC, surgery might be considered in stage II to IIIA if the patient strongly insists that surgery should be performed first. However, TNBC has a higher locoregional recurrence (LRR) and visceral metastases than other subtypes of breast cancer [6-8]. Therefore, there is a concern that mastectomy should be considered in patients with TNBC. Several studies have investigated whether BCS achieved better outcomes than mastectomy, but the results are controversial [9-12]. We compared the outcomes of BCS and mastectomy in patients with stage II-IIIA TNBC. Furthermore, we assessed prognostic factors for recurrence or metastasis.
METHODSPatientsThe patients were diagnosed with primary TNBC and treated at Pusan National University Hospital and Pusan National University Yangsan Hospital from 2010 to 2014. The patients were treated with surgery and adjuvant chemotherapy, or radiation therapy. Patients with ductal carcinoma in situ, stage I, IIIB, and IIIC, recurrent breast cancer, metastases at diagnosis, or neoadjuvant chemotherapy were excluded.
Age at diagnosis was used to analyze the survival period. We assessed the type of surgery, histology, tumor size, lymph node status, tumor grade, type of chemotherapy, and radiation therapy. In the BCS group, an intraoperative frozen biopsy was obtained from the remnant breast tissue, and additional breast tissue was resected when the frozen biopsy results were positive. The surgical margin was assessed as negative when the final pathologic report confirmed that “no tumor was present”.
Hormone receptor status was graded using Allred scoring and scores of 3-8 were considered positive. TNBC included tumors that were negative for hormone receptors and human epidermal growth factor receptor 2 (HER2). The cancers were staged according to the breast cancer guidelines of the 8th American Joint Committee on Cancer (AJCC).
We monitored patient status every three to six months for the first five years after primary therapy and annually thereafter. The date and cause of death were obtained from the medical records of the deceased patients. LRR was defined as recurrence in the ipsilateral breast, chest wall or axillary, supraclavicular, infraclavicular, or internal mammary nodes. Metastasis in any other organ was considered a distant metastasis. LRR-free survival (LRRFS), disease-free survival (DFS), and overall survival (OS) were measured from the date of surgery to the date of LRR, the time of recurrence or metastasis, and either death or the date the patient last visited the outpatient clinic, respectively.
This study was approved by the Institutional Review Board of Pusan National University, Korea (IRB No. 04-2020-011).
Statistical analysesThe demographics and tumor characteristics were compared between the BCS and mastectomy groups using Chi-squared tests. If a variable with a significant difference between the two groups was a factor that affected LRR or metastasis, we performed an adjusted multivariate logistic regression analysis. The Kaplan-Meier method was used for the LRRFS, DFS, and OS comparisons by the log-rank test, and p< 0.050 was considered significant. The Cox proportional hazards model was used to select significant covariates. The data were analyzed using SPSS version 21 (SPSS Inc., Chicago, USA).
RESULTSAltogether, 172 patients diagnosed with stage II-IIIA TNBC from 2010 to 2014 were included in this study. The median age of the patients was 51 years (range, 22-82 years). One hundred and one (58.7%) patients underwent BCS, while 71 (41.3%) underwent mastectomy. The patient and tumor characteristics between the two groups are summarized in Table 1. Compared to the BCS group, the mastectomy group had a higher tumor stage (T stage), more stage IIIA patients, and a lower proportion of Ki-67. In the BCS group, 97.0% of the patients received radiotherapy compared to 62.0% of the patients in the mastectomy group (p< 0.001). Of the three patients (3.0%) in the BCS group who did not receive adjuvant radiotherapy, two patients refused radiotherapy, and one developed a LRR before the planned radiotherapy. One hundred (99.0%) patients in the BCS group received chemotherapy compared to 64 (90.1%) patients in the mastectomy group (p= 0.007).
Overall, 31 patients (18.0%) had a LRR or distant metastasis with a median follow-up period of 71 months (range, 6-113 months). LRR occurred in 18 patients: eleven in the BCS group and seven in the mastectomy group. Distant metastases was seen in 22 patients: 12 in the BCS group and 10 in the mastectomy group.
The 5-year LRR-free, disease-free, and overall survival rates of the BCS versus mastectomy groups were 93.8% vs. 95.3%, 89.8% vs. 90.7%, and 90.8% vs. 86.3%, respectively, but the differences were not statistically significant. Figure 1 shows the Kaplan-Meier curves for LRRFS, DFS, and OS according to the differences in surgical methods.
The absence of lymphovascular invasion (LVI) was significantly associated with superior DFS on multivariate analysis (p = 0.047). As shown in Table 2, advanced stage was significantly associated with OS on multivariate analysis, but there were no significant factors affecting LRRFS in multivariate analysis.
The tumor stage (T stage) could be a confounding variable because the pT stage was higher in the mastectomy group than in the BCS group. Therefore, we performed an adjusted multivariate logistic regression analysis according to the pT stage. However, as shown in Table 3, whether the T stage was classified as T1, T2, and T3, or classified as group T1 and T2 combined, and group T3, the difference in surgical method did not affect LRR or metastasis.
DISCUSSIONMastectomy is often preferred over BCS because of the aggressive features of TNBC. However, some studies have demonstrated that BCS has better outcomes than mastectomy in the early stages of TNBC [12-15]. Therefore, this study excluded stage I TNBC when comparing the results of BCS and mastectomy. In addition, we excluded patients who received neoadjuvant chemotherapy to obtain accurate pathological results. If patients are diagnosed with stage II to IIIA breast cancer after surgery following neoadjuvant chemotherapy, it would be difficult to accurately compare them to the patient groups in this study because of the debulking effect of neoadjuvant chemotherapy. When breast cancer has penetrated the skin, or there are multiple lymph node metastases on preoperative examination, neoadjuvant chemotherapy should be considered first [16]. Therefore, patients with stage IIIB and IIIC breast cancer were excluded to minimize bias from these selections. As mentioned in the results of this study, BCS was not inferior to mastectomy, and the results were similar to those of previous studies [17-19]. However, the limitations of the study by Chen et al. [18] were inadequate follow-up duration and the lack of available information on adjuvant chemotherapy or neoadjuvant chemotherapy. A meta-analysis by Wang et al. [19], indicated that BCS showed an improvement in LRR compared to mastectomy, but this study did not perform stage-related comparisons. In the current study, the median age of the patients was 51 years, which was consistent with other research reports showed that Korean women in their mid-forties had a high breast cancer incidence rate, unlike the rate in Western countries [20,21]. Therefore, BCS is performed more frequently than mastectomies. The BCS methods included a quadrantectomy with a local flap, latissimus dorsi musculocutaneous flap (LDMCF) with or without saline bag insertion, and reduction mammoplasty. We performed quadrantectomy to ensure sufficient margins in the BCS group. As mentioned in the methods section, when the intraoperative frozen biopsy margin was positive, breast tissue was resected until the biopsy was confirmed as negative. However, there were two cases in which atypical cells were identified in the final pathological results, and these patients were treated with radiotherapy. Mastectomy included total mastectomy, nipple-sparing mastectomy, and skin-sparing mastectomy with or without axillary lymph node dissection. All except three of the patients in the BCS group received adjuvant radiotherapy, and 6.2% of the patients in this study had LRR at five years after BCS. Considering that 4.7% of the patients in the mastectomy group had LRR after five years, BCS with radiotherapy followed by adjuvant chemotherapy achieved good results in patients with stage II-IIIA TNBC. Although radiotherapy was not completely excluded in the mastectomy group, adjuvant radiotherapy could significantly reduce the risk of LRR in TNBC [22]. Further, except for one patient, adjuvant chemotherapy was performed more often in the BCS group (Table 1). In the mastectomy group, out of seven patients who did not received adjuvant chemotherapy, there was one patient with LRR and one with liver metastasis. A 55-year-old patient refused chemotherapy and radiotherapy after mastectomy because of a psychiatric problem, and a 69-year-old patient refused both treatments after mastectomy. In this study, multivariate analyses showed that chemotherapy was not a significant prognostic factor. In addition, the presence of LVI was related to poor prognosis, and this result has further been reported in other studies [14,17,23,24]. Therefore, more intensive treatment should be considered for TNBC patients with LVI.
This study had several limitations due to its retrospective design. Radiotherapy, chemotherapy, the value of Ki-67, and pT stage were different between the two groups, and selection biases were not completely resolved. In particular, the higher the pT stage, the more often mastectomy was performed compared to BCS (Table 1). Since the pT stage was a confounding variable that could affect outcomes such as LRR, we conducted an adjusted multivariate logistic regression analysis. As a result, though we included the pT stage in the model and adjusted for the pT stage, the difference in surgery type did not affect LRR or metastasis (Table 3).
Despite these limitations, the follow-up period was relatively long. As in other studies, there was no significant difference in the outcomes between BCS and mastectomy in the advanced stages [10,17]. Although neoadjuvant chemotherapy is considered as the first treatment option for advanced stage triple negative breast cancer, BCS could be selected if patients want to undergo surgery first even in the advanced stage.
In conclusion, BCS could be considered in patients with advanced TNBC patients because it has been shown that the outcomes of BCS are not inferior to those of mastectomy.
ACKNOWLEDGMENTSThe authors thank Mihyang Ha, M.S. form the Interdisciplinary Program of Genomic Science, Pusan National University for her statistical analysis.
Figure 1.Locoregional recurrence-free survival (A) disease-free survival (B), and overall survival (C) curves according to the treatment.
BCS=breast conservation surgery.
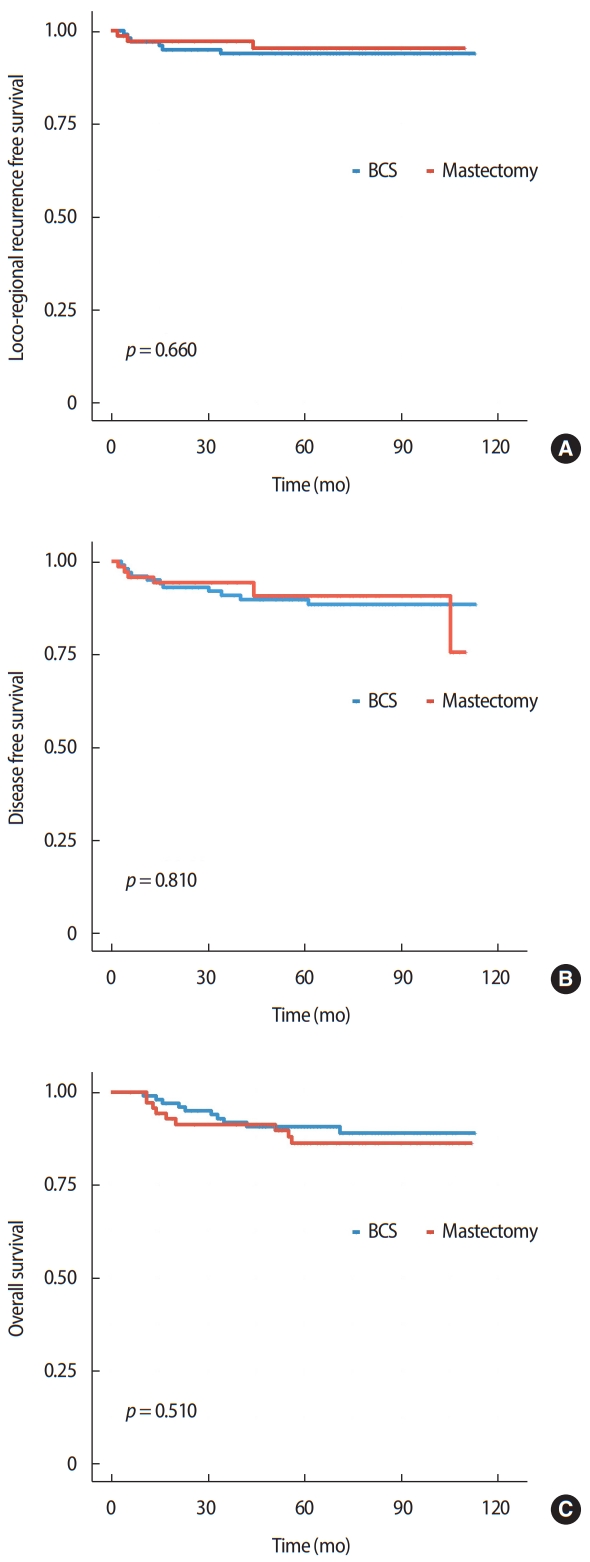
Table 1.Association between characteristics of patients and surgical type Table 2.Multivariate cox proportional hazards regression for LRRFS, DFS, and OS Table 3.Adjusted multivariate logistic regression of pathologic T stage and surgery type for LRRFS, DFS, and OS
(A) T stage was classified as T1, T2, T3, respectively
REFERENCES1. Fitzal F, Riedl O, Mittlböck M, Dubsky P, Bartsch R, Steger G, et al. Oncologic safety of breast conserving surgery after tumour downsizing by neoadjuvant therapy: a retrospective single centre cohort study. Breast Cancer Res Treat 2011;127:121-8.
2. Golshan M, Cirrincione CT, Sikov WM, Berry DA, Jasinski S, Weisberg TF, et al. Impact of neoadjuvant chemotherapy in stage II-III triple negative breast cancer on eligibility for breast-conserving surgery and breast conservation rates: surgical results from CALGB 40603 (Alliance). Ann Surg 2015;262:434-9.
3. Golshan M, Cirrincione CT, Sikov WM, Carey LA, Berry DA, Overmoyer B, et al. Impact of neoadjuvant therapy on eligibility for and frequency of breast conservation in stage II-III HER2-positive breast cancer: surgical results of CALGB 40601 (Alliance). Breast Cancer Res Treat 2016;160:297-304.
4. Mo H, Kim Y, Rhu J, Lee KH, Kim TY, Im SA, et al. Actual conversion rate from total mastectomy to breast conservation after neoadjuvant chemotherapy for stages II–III breast cancer patients. J Breast Dis 2017;5:51-6.
5. Chintamani , Gogne A, Khandelwal R, Tandon M, Jain S, Kumar Y, et al. The correlation of anxiety and depression levels with response to neoadjuvant chemotherapy in patients with breast cancer. JRSM Short Rep 2011;2:1-5.
6. Ihemelandu CU, Naab TJ, Mezghebe HM, Makambi KH, Siram SM, Leffall LD Jr, et al. Treatment and survival outcome for molecular breast cancer subtypes in black women. Ann Surg 2008;247:463-9.
7. Lowery AJ, Kell MR, Glynn RW, Kerin MJ, Sweeney KJ. Locoregional recurrence after breast cancer surgery: a systematic review by receptor phenotype. Breast Cancer Res Treat 2012;133:831-41.
8. McGuire A, Lowery AJ, Kell MR, Kerin MJ, Sweeney KJ. Locoregional recurrence following breast cancer surgery in the trastuzumab era: a systematic review by subtype. Ann Surg Oncol 2017;24:3124-32.
9. Nguyen PL, Taghian AG, Katz MS, Niemierko A, Abi Raad, Boon WL, et al. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol 2008;26:2373-8.
10. Parker CC, Ampil F, Burton G, Li BD, Chu QD. Is breast conservation therapy a viable option for patients with triple-receptor negative breast cancer? Surgery 2010;148:386-91.
11. Solin LJ, Hwang WT, Vapiwala N. Outcome after breast conservation treatment with radiation for women with triple-negative early-stage invasive breast carcinoma. Clin Breast Cancer 2009;9:96-100.
12. Zumsteg ZS, Morrow M, Arnold B, Zheng J, Zhang Z, Robson M, et al. Breast-conserving therapy achieves locoregional outcomes comparable to mastectomy in women with T1-2N0 triple-negative breast cancer. Ann Surg Oncol 2013;20:3469-76.
13. Hwang ES, Lichtensztajn DY, Gomez SL, Fowble B, Clarke CA. Survival after lumpectomy and mastectomy for early stage invasive breast cancer: the effect of age and hormone receptor status. Cancer 2013;119:1402-11.
14. Kim K, Park HJ, Shin KH, Kim JH, Choi DH, Park W, et al. Breast conservation therapy versus mastectomy in patients with T1-2N1 triple-negative breast cancer: pooled analysis of KROG 14-18 and 14-23. Cancer Res Treat 2018;50:1316-23.
15. Vila J, Gandini S, Gentilini O. Overall survival according to type of surgery in young (≤40 years) early breast cancer patients: a systematic meta-analysis comparing breast-conserving surgery versus mastectomy. Breast 2015;24:175-81.
16. Liedtke C, Mazouni C, Hess KR, André F, Tordai A, Mejia JA, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol 2008;26:1275-81.
17. Adkins FC, Gonzalez-Angulo AM, Lei X, Hernandez-Aya LF, Mittendorf EA, Litton JK, et al. Triple-negative breast cancer is not a contraindication for breast conservation. Ann Surg Oncol 2011;18:3164-73.
18. Chen QX, Wang XX, Lin PY, Zhang J, Li JJ, Song CG, et al. The different outcomes between breast-conserving surgery and mastectomy in triple-negative breast cancer: a population-based study from the SEER 18 database. Oncotarget 2017;8:4773-80.
19. Wang J, Xie X, Wang X, Tang J, Pan Q, Zhang Y, et al. Locoregional and distant recurrences after breast conserving therapy in patients with triple-negative breast cancer: a meta-analysis. Surg Oncol 2013;22:247-55.
20. Lee SK, Kim SW, Yu JH, Lee JE, Kim JY, Woo J, et al. Is the high proportion of young age at breast cancer onset a unique feature of Asian breast cancer? Breast Cancer Res Treat 2019;173:189-99.
21. Yoo KY, Kang D, Park SK, Kim SU, Shin A, Yoon H, et al. Epidemiology of breast cancer in Korea: occurrence, high-risk groups, and prevention. J Korean Med Sci 2002;17:1-6.
22. O’Rorke MA, Murray LJ, Brand JS, Bhoo-Pathy N. The value of adjuvant radiotherapy on survival and recurrence in triple-negative breast cancer: a systematic review and meta-analysis of 5507 patients. Cancer Treat Rev 2016;47:12-21.
|
|
||||||||||||||||||||||||||||||||||