AbstractPurposeAccording to the American Joint Committee on Cancer’s 8th Edition Manual, lobular carcinoma in situ (LCIS) is no longer considered a malignant disease, although it may be a precursor to the development of breast cancer. The present study aimed to evaluate the clinicopathological features and prognosis of LCIS.
MethodsThis study retrospectively analyzed clinicopathological features and prognosis data of LCIS among patients who underwent breast surgery at Severance Hospital, Seoul, South Korea from 1991 to 2016.
ResultsOf the 47 patients, 49 cases of LCIS were confirmed by postoperative pathology. The mean patient age was 48.15±8.34 years. Most patients (81.6%) did not have palpable tumors at diagnosis, and 51.0% showed no microcalcification on mammography. Breast-conserving surgery was performed more frequently than total mastectomy (77.6% vs. 22.4%). The mean tumor size was 1.63±2.11 cm. There were only 3 cases of pleomorphic LCIS. Hormone receptor-positive tumors were noted in 47 cases, however, the hormone receptor status was unknown in the other 2 cases. There were no LCIS recurrences or deaths during the follow-up period (mean 56 months).
INTRODUCTIONLobular carcinoma in situ (LCIS) is a neoplasm of the breast, which can affect multiple lobules and terminal ducts with the expansion of proliferative atypical cells [1,2]. In LCIS, there are abnormal monomorphic small round cells that have no polarity, and are detached from each other [1,2]. Although these cells fill the lobular spaces and extend into the adjacent terminal ducts, LCIS is characterized by conformance of the abnormal cells to the outline of the normal lobules [1,2].
Similarly, atypical lobular hyperplasia (ALH) is characterized by the proliferation of monomorphic epithelial cells, which expand up to 50% of the terminal duct lobular units [3,4]. The distinction between ALH and LCIS may be ambiguous and subjective, as the diagnosis is based on quantitative differences in the extent of lobular involvement [5]. Lobular neoplasia (LN) refers to the coexistence of LCIS and ALH, and is associated with an increased risk of developing invasive carcinoma in either breast, suggesting that LN is a non-obligate precursor to breast cancer, and not merely a risk factor [2,6,7]. Traditionally, when a core needle biopsy reveals a diagnosis of LN, tumor excision is recommended. However, the management of LN is controversial, and some authors recommend observation rather than surgical excision.
When the breast cancer staging system was revised in 2017, LCIS was excluded from the “malignant” category [8]. Furthermore, the recommended treatment for LCIS, when diagnosed by core needle biopsy, was revised from surgical excision to risk management [9]. Surgical excision can be considered in cases of pleomorphic LCIS, or LCIS that is non-concordant with imaging studies, while follow-up with close observation is the primary course of action in cases of classic LCIS or LCIS which is concordant with imaging studies [9]. However, no previous studies have evaluated the prognosis and clinicopathologic features of LCIS in South Korean women. Therefore, the present study aimed to evaluate LCIS in South Korean women.
METHODSWe retrospectively reviewed electronic medical records and the breast cancer registry database of Severance Hospital, Yonsei University health system. The breast cancer registry includes information about the clinical characteristics of patients, treatment methods, pre- and postoperative pathologic findings, preoperative physical examination results, disease recurrence, mortality, and follow-ups [10].
We included in this study patients who underwent breast surgery for LCIS at Severance Hospital between January 1991 and December 2016. Definitive surgery for LCIS was performed in 73 cases that were diagnosed pre- or postoperatively. We excluded 9 cases that were diagnosed with LCIS preoperatively, but were later diagnosed with invasive cancer or ductal carcinoma in situ (DCIS) postoperatively; 13 cases of LCIS with contralateral DCIS or invasive carcinoma; and 2 cases of LCIS combined with DCIS (Figure 1). Because contralateral DCIS or invasive carcinoma can affect the prognosis, those cases were excluded. A total of 49 cases (breasts) from 47 patients, including 2 patients with synchronous LCIS, were included in the study. We reviewed and analyzed the clinicopathologic features and prognosis of LCIS for all 49 cases. The clinicopathologic features included the patient’s age, physical examination findings, imaging findings, pathologic findings, treatment methods, and survival outcomes. A preoperative physical examination was performed by experienced surgeons, and when applicable, a palpable mass was described, with or without information on the location or size of the lesion, in the medical database. Preoperative imaging evaluations, including mammography, ultrasonography, and magnetic resonance imaging (MRI), were reported by experienced radiologists. The initial findings of the preoperative imaging studies were analyzed in conjunction with the final pathological findings to assess the correlation between imaging and pathology.
Patients underwent breast-conserving surgery or mastectomy according to the surgeons’ decision based on the tumor size, location, and multiplicity and the patient’s preference. After surgery, some patients who underwent breast-conserving surgery received adjuvant radiotherapy according to a multidisciplinary team approach or shared decision-making. Follow-up every 6 months was recommended for those who underwent surgery.
The final pathological findings were reviewed to analyze histopathological variables, including tumor size, hormone receptor status, E-cadherin expression, and pleomorphism. Estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2)/neu expression were evaluated using immunohistochemical staining of formalin-fixed, paraffin-embedded whole sections from surgically resected breast specimens. The cutoff value for ER and PR positivity was >1% for immunohistochemistry staining. Pleomorphism was assessed by a breast pathologist, and was classified as either absent or present.
Statistical analysisUsing data for 49 cases from 47 patients, we analyzed categorical variables using the chi-square test or Fisher’s exact test. Continuous variables were analyzed using the Student’s t-test. The significant factors estimated by the aforementioned analyses were confirmed using univariate logistic regression. P-values <0.05 were considered significant, and all tests were two-sided. Statistical analyses were performed using commercially available statistical software, SPSS Statistics 24 (IBM Corp., Armonk, USA).
RESULTSThe clinicopathologic features for all cases are shown in Table 1. The mean onset of LCIS age was 48.15±8.34 years. About two-thirds of the patients (30/47, 63.8%) were under the age of 50. Upon physical examination, a majority of the cases (40/49, 81.6%) were not palpable. The most common biopsy performed for preoperative diagnosis was core needle biopsy, followed by vacuum-assisted breast biopsy and excisional biopsy. Approximately one-third of the cases (18/49, 36.7%) showed microcalcification on mammography. More than half of the cases (28/49, 57.1%) showed no mass or nodule on ultrasonography. The most common breast imaging reporting and data system (BI-RADS) classification was category 4 (35/49, 71.4%). MRI enhancement was observed in 19 of 49 cases (38.8%). Right-sided tumors were slightly more frequent than left-sided tumors (44.9% vs. 55.1%).
Breast-conserving surgery was performed more frequently than total mastectomy (77.6% vs. 22.4%). The median and mean tumor sizes were 0.8 cm (range, 0.1–9.7 cm) and 1.63±2.11 cm, respectively. Approximately two-thirds of the cases (33/49, 67.3%) had a tumor size of ≤2 cm. E-cadherin expression was negative in 83.7% of the cases. Only 3 cases (6.1%) were diagnosed with pleomorphic LCIS. The rates of ER and PR positivity were 95.9% and 63.3%, respectively. More than half of the cases (26/49, 53.1%) did not show HER2 overexpression.
None of the patients received adjuvant chemotherapy. Hormone therapy was administered to 80.9% (38/47) of the patients. Radiotherapy was performed in 38.8% (19/49) of the patients, who also underwent breast-conserving surgery.
For the survival analysis, the median follow-up period was 56 months (range, 8-203 months). One patient died due to thyroid cancer with liver and lung metastases, therefore the death was not attributed to LCIS (Figure 2). There was no recurrence of or mortality associated with pure LCIS.
DISCUSSIONThe results of the present study demonstrated that women with LCIS have excellent oncologic outcomes, with no recurrence or subsequent invasive cancer development over a 5 year period after excision surgery. Previous studies have shown that 7%–15% of women with surgically removed LCIS subsequently developed invasive cancer within 10 years of surgical excision [11,12]. This discrepancy may be due to differences in the sample size and follow-up periods between the studies. Furthermore, the use of tamoxifen may contribute to differences in the development of subsequent invasive cancer in women with a history of LCIS. In the present study, the majority of patients received tamoxifen therapy, which effectively reduces the incidence of not only subsequent invasive cancer, but also contralateral breast cancer [13,14].
The median age at onset of LCIS for the present study was 48 years. Additionally, there were more women aged≤50 years than >50 years. In previous studies, the mean and median onset age of LCIS were 44–49 years [11,15] and 45–49 years [12], respectively. The onset age of LCIS in the present study was slightly lower than the median age (50 years) of patients with breast cancer in the Korean Breast Cancer Registry [16], and was concordant with the age of onset reported in previous studies [11,12].
In the current study, more than half of the women showed no abnormalities on physical examinations, with only 9 cased being palpable. According to a previous study, most cases of LCIS are incidentally detected on breast core needle biopsies or a surgical excisions of specimens targeting other lesions [1]. Our findings support this suggestion. Additionally, the results of the present study showed that 61% of women whose MRI findings were available had signal enhancement on their scans, which is a finding suspicious for LCIS. This suggests that MRI can be helpful in detecting LCIS that may be occult in mammography and breast ultrasonography. However, further studies are needed to evaluate the role of breast MRI in detecting LCIS.
Bilaterality is traditionally believed to be a common characteristic of LCIS [15]. However, in the present study, only 2 patients had bilateral LCIS (4.25%). This may have been due to differences in the biopsy methods used in the studies. Beute et al. [15] reviewed 165 breasts from 119 patients with LCIS who underwent surgery between 1974 and 1987. They performed mirror-image biopsies or contralateral mastectomies, which are not currently widely used to treat women with LCIS, in 82 patients. This may have contributed to the differences in LCIS bilaterality between the study by Beute et al. [15] and the present study. In a recent study of 1060 women with LCIS, 2% had synchronous bilateral LCIS [17]. Another study reported a similar rate of 2.1% for synchronous bilateral LCIS [18]. These results are similar to those of the present study.
There is no consensus on management strategies for LCIS [19,20]. Recent guidelines recommend that risk management, including close follow-up, risk-reducing medication, or preventative mastectomy, should be considered for women with LCIS [9]. Routine surgical excision, however, is not recommended for the management of LCIS. Because the current study enrolled women who underwent surgery prior to 2017, the patients were not actively followed-up with, as routine surveillance was not recommended at that time. Further studies are needed to evaluate the effects of close follow-up without surgery in the management plan for LCIS, based on the updated guidelines.
According to a large cohort study previously conducted using the National Cancer Institute’s surveillance, epidemiology, and end results database, the overall survival of the group who underwent radiation therapy after breast-conserving surgery was found to be a little bit longer than the lumpectomy-only group [19], even though radiation therapy is not routinely recommended for LCIS. On the other hand, the results of the present study showed an excellent prognosis for LCIS, whether the patient was treated with or without radiation therapy. There is still not sufficient evidence to evaluate the impact of radiation therapy for the treatment of LCIS.
Pleomorphic LCIS is considered an aggressive type of LCIS. In contrast to classic LCIS, pleomorphic LCIS has distinctive pathologic features, including: high-grade cytologic features, comedo-type necrosis, florid growth pattern, calcification, abundant cytoplasm, prominent nucleoli, and irregular nuclear membranes [2]. In the present study, pleomorphic LCIS was found in 3 cases (6.1%), which was lower than what was found in a previously reported study [21]. The low incidence rate of pleomorphic LCIS may be due to differences in sample sizes, ethnicities, and proportion of excisional biopsies. Because previous large cohort studies lacked information on pathologic variants of LCIS [17,19], further large cohort studies including information on pathological variants are needed to verify the true incidence rate of pleomorphic LCIS.
Finally, because of the nature of retrospective studies, the present study has methodological limitations. The small number of patients is a major limitation. However, this study includes recent data regarding the clinicopathologic features and prognosis of South Korean women with LCIS. To the best of our knowledge, the median follow-up period of 56-months is also the longest follow-up period in studies of women with LCIS in South Korea. A homogenous patient population from a single institution is also a strength of this study. However, large multicenter studies are needed to provide more insight into the management of LCIS.
Figure 1.Schematic of patient selection for the study.
LCIS=lobular carcinoma in situ; DCIS=ductal carcinoma in situ.
Figure 2.Overall survival of pure LCIS. One patient died due to thyroid cancer combined with liver and lung metastases; the death was not attributed to LCIS. There was no recurrence of or mortality associated with pure LCIS. LCIS=lobular carcinoma in situ; OS=overall survival. 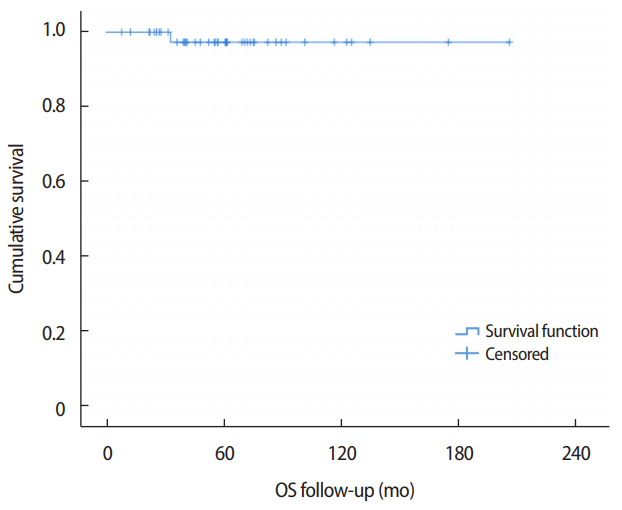
Table 1.Clinicopathological features and prognosis of LCIS Values are presented as mean±standard deviation or number (%). LCIS=lobular carcinoma in situ; US=ultrasonography; BI-RADS=breast imaging reporting and data system; MRI=magnetic resonance imaging; N/A=not available; ER=estrogen receptor; PR=progesterone receptor; HER2=human epidermal growth factor receptor 2. REFERENCES2. Ginter PS, D’Alfonso TM. Current concepts in diagnosis, molecular features, and management of lobular carcinoma in situ of the breast with a discussion of morphologic variants. Arch Pathol Lab Med 2017;141:1668-78.
3. Muller KE, Roberts E, Zhao L, Jorns JM. Isolated atypical lobular hyperplasia diagnosed on breast biopsy: low upgrade rate on subsequent excision with long-term follow-up. Arch Pathol Lab Med 2018;142:391-5.
5. Logan GJ, Dabbs DJ, Lucas PC, Jankowitz RC, Brown DD, Clark BZ, et al. Molecular drivers of lobular carcinoma in situ. Breast Cancer Res 2015;17:76.
6. O’Malley FP. Lobular neoplasia: morphology, biological potential and management in core biopsies. Mod Pathol 2010;23:S14-25.
7. Racz JM, Carter JM, Degnim AC. Lobular neoplasia and atypical ductal hyperplasia on core biopsy: current surgical management recommendations. Ann Surg Oncol 2017;24:2848-54.
8. Giuliano AE, Connolly JL, Edge SB, Mittendorf EA, Rugo HS, Solin LJ, et al. Breast cancer-major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 2017;67:291-303.
9. National comprehensive cancer network guidelines. Breast cancer version 1. 2018. National Comprehensive Cancer Network. https://www2.tri-kobe.org/nccn/guideline/archive/breast2018/english/breast_v1.pdf. Accessed May 25th, 2021.
10. Park S, Park BW, Kim TH, Jeon CW, Kang HS, Choi JE, et al. Lack of either estrogen or progesterone receptor expression is associated with poor survival outcome among luminal A breast cancer subtype. Ann Surg Oncol 2013;20:1505-13.
11. McDivitt RW, Hutter RV, Foote FW Jr., Stewart FW. In situ lobular carcinoma. A prospective follow-up study indicating cumulative patient risks. JAMA 1967;201:82-6.
12. Chuba PJ, Hamre MR, Yap J, Severson RK, Lucas D, Shamsa F, et al. Bilateral risk for subsequent breast cancer after lobular carcinoma-in-situ: analysis of surveillance, epidemiology, and end results data. J Clin Oncol 2005;23:5534-41.
13. Coopey SB, Mazzola E, Buckley JM, Sharko J, Belli AK, Kim EM, et al. The role of chemoprevention in modifying the risk of breast cancer in women with atypical breast lesions. Breast Cancer Res Treat 2012;136:627-33.
14. Cuzick J, Sestak I, Bonanni B, Costantino JP, Cummings S, DeCensi A, et al. Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet 2013;381:1827-34.
15. Beute BJ, Kalisher L, Hutter RV. Lobular carcinoma in situ of the breast: clinical, pathologic, and mammographic features. AJR Am J Roentgenol 1991;157:257-65.
16. Kang SY, Kim YS, Kim Z, Kim HY, Lee SK, Jung KW, et al. Basic findings regarding breast cancer in Korea in 2015: data from a breast cancer registry. J Breast Cancer 2018;21:1-10.
17. King TA, Pilewskie M, Muhsen S, Patil S, Mautner SK, Park A, et al. Lobular carcinoma in situ: a 29-year longitudinal experience evaluating clinicopathologic features and breast cancer risk. J Clin Oncol 2015;33:3945-52.
18. Shin H, Lee HB, Han W, Noh DY, Moon HG. The roles of modern breast imaging techniques for evaluation of lobular carcinoma in situ of the breast. J Breast Dis 2015;3:71-6.
19. Cheng P, Huang Q, Shou J, Hu G, Han M, Huang J. Treatment and survival outcomes of lobular carcinoma in situ of the breast: a SEER population based study. Oncotarget 2017;8:103047-54.
|
|
||||||||||||||||||||||||||||||||||